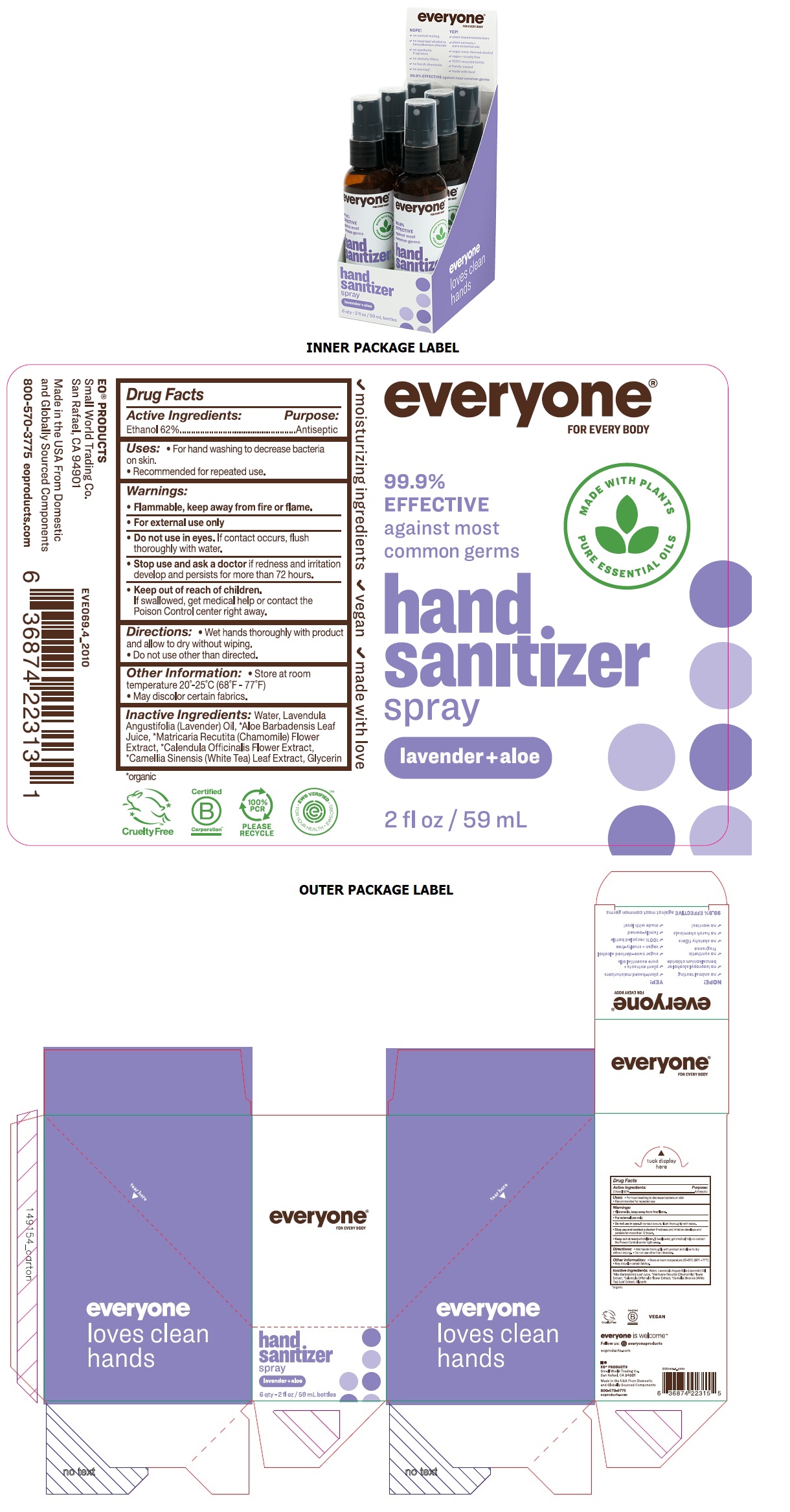
Label: EVERYONE HAND SANITIZER LAVENDER ALOE- ethyl alcohol spray
- NDC Code(s): 54748-602-05, 54748-602-09
- Packager: EO Products, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients:
- Purpose:
- Uses:
- Warnings:
- Directions:
- Other Information:
- Inactive Ingredients:
-
SPL UNCLASSIFIED SECTION
everyone® FOR EVERY BODY
everyone loves clean hands
99.9%EFFECTIVE against most common germs
MADE WITH PLANTS
PURE ESSENTIAL OILS
Cruelty Free
√ moisturizing ingredients
√ vegan
√ made with love
NOPE!
√ no animal testing
√ no isopropyl alcohol or benzalkonium chloride
√ no synthetic fragrance
√ no sketchy fillers
√ no harsh chemicals
√ no worries!
YEP!
√ plant-based moisturizers
√ plant extracts + pure essential oils
√ sugar cane-derived alcohol
√ vegan + cruelty-free
√ 100% recycled bottle
√ family-owned
√ made with love!
everyone is welcome™
Follow us: everyoneproducts
EO® PRODUCTS
Small world Trading Co.
San Rafael, CA 94901
Made in the USA From Domestic and Globally Sourced Components
800-570-3775 eoproducts.com
- Packaging
-
INGREDIENTS AND APPEARANCE
EVERYONE HAND SANITIZER LAVENDER ALOE
ethyl alcohol sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54748-602 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) LAVENDER OIL (UNII: ZBP1YXW0H8) ALOE VERA LEAF (UNII: ZY81Z83H0X) CHAMOMILE (UNII: FGL3685T2X) CALENDULA OFFICINALIS FLOWER (UNII: P0M7O4Y7YD) WHITE TEA (UNII: O0M3396E09) GLYCERIN (UNII: PDC6A3C0OX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54748-602-09 6 in 1 BOX 01/25/2021 1 NDC:54748-602-05 59 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 01/25/2021 Labeler - EO Products, LLC (786611210) Establishment Name Address ID/FEI Business Operations EO Products, LLC 786611210 manufacture(54748-602)