Label: SODIUM CHLORIDE- sodium chloride injection, solution
-
NDC Code(s):
17271-701-02,
17271-701-03,
17271-701-05,
17271-701-06, view more17271-701-07
- Packager: Becton Dickinson and Company
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 21, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
0.9% Sodium Chloride Injection, USP solution is sterile and nonpyrogenic. It is a parenteral solution containing sodium chloride in water for injection intended for intravenous administration.
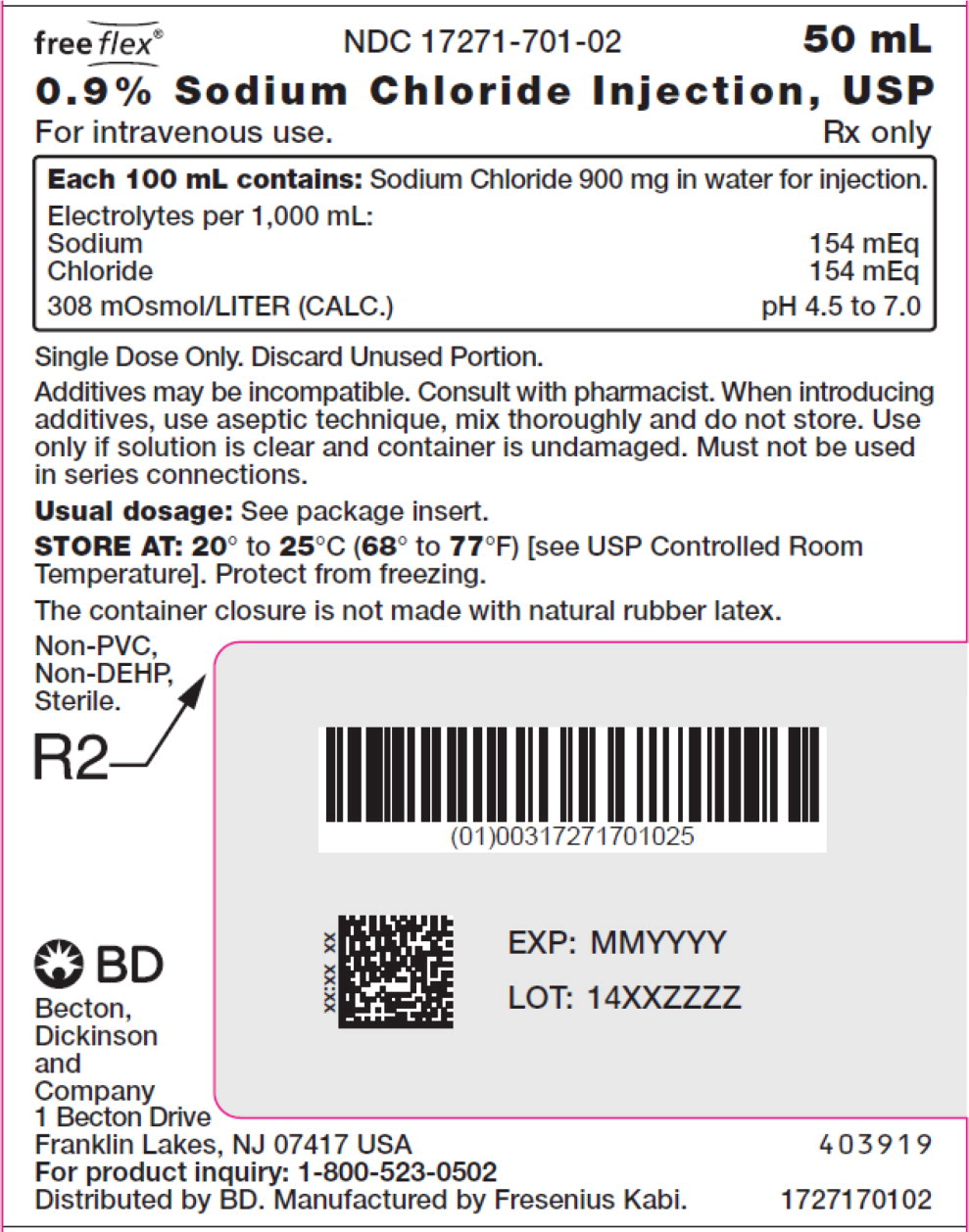
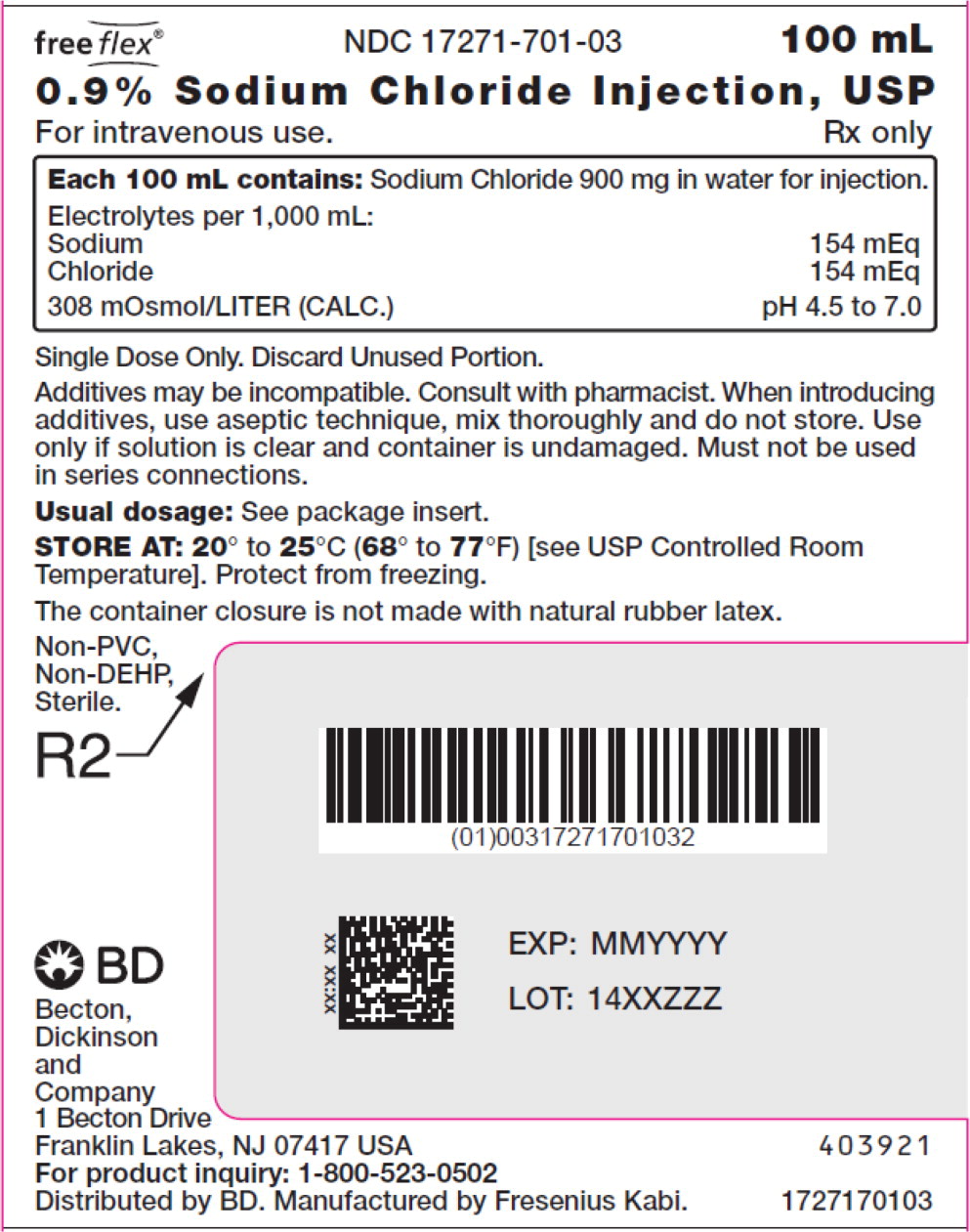
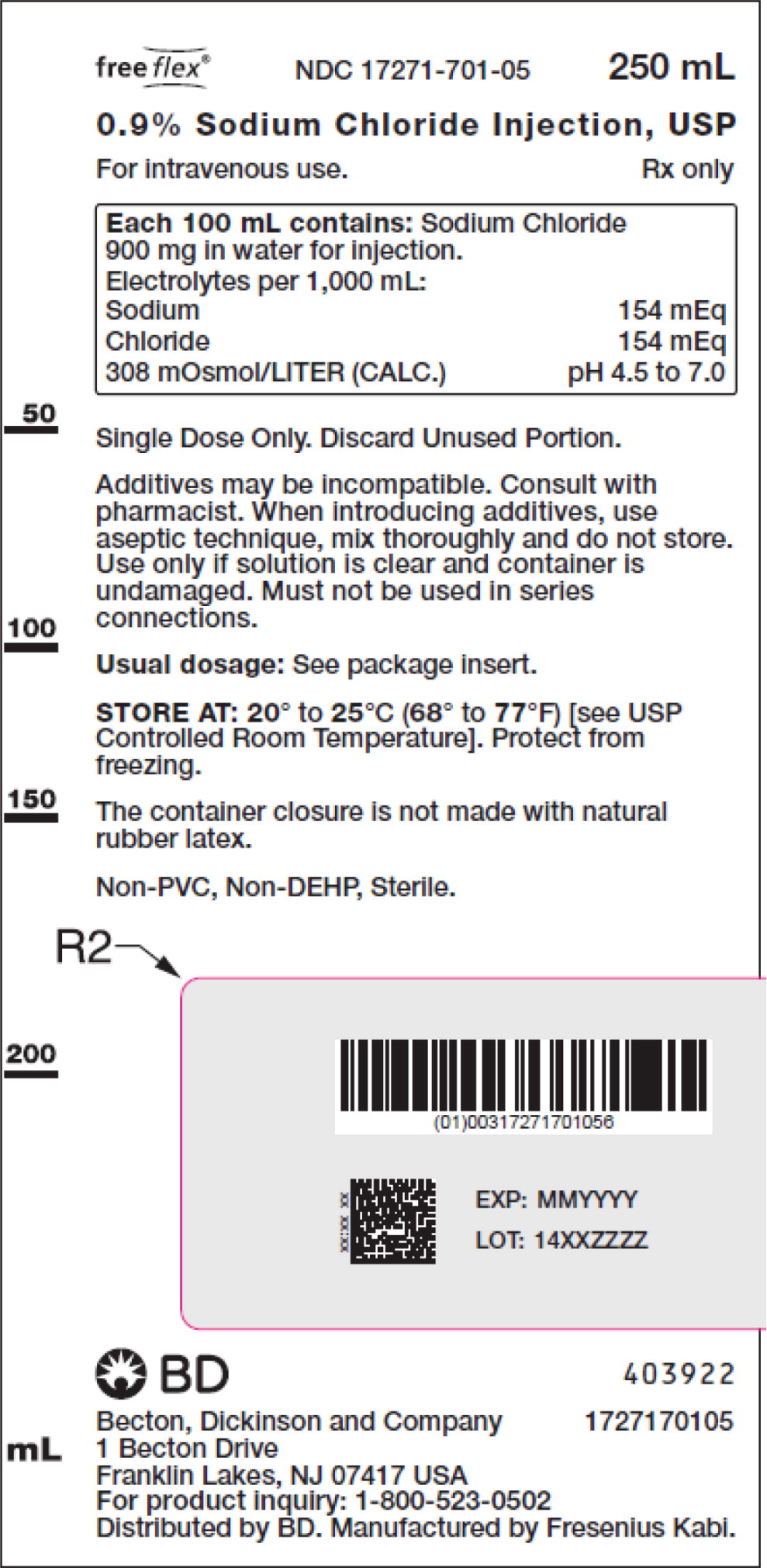
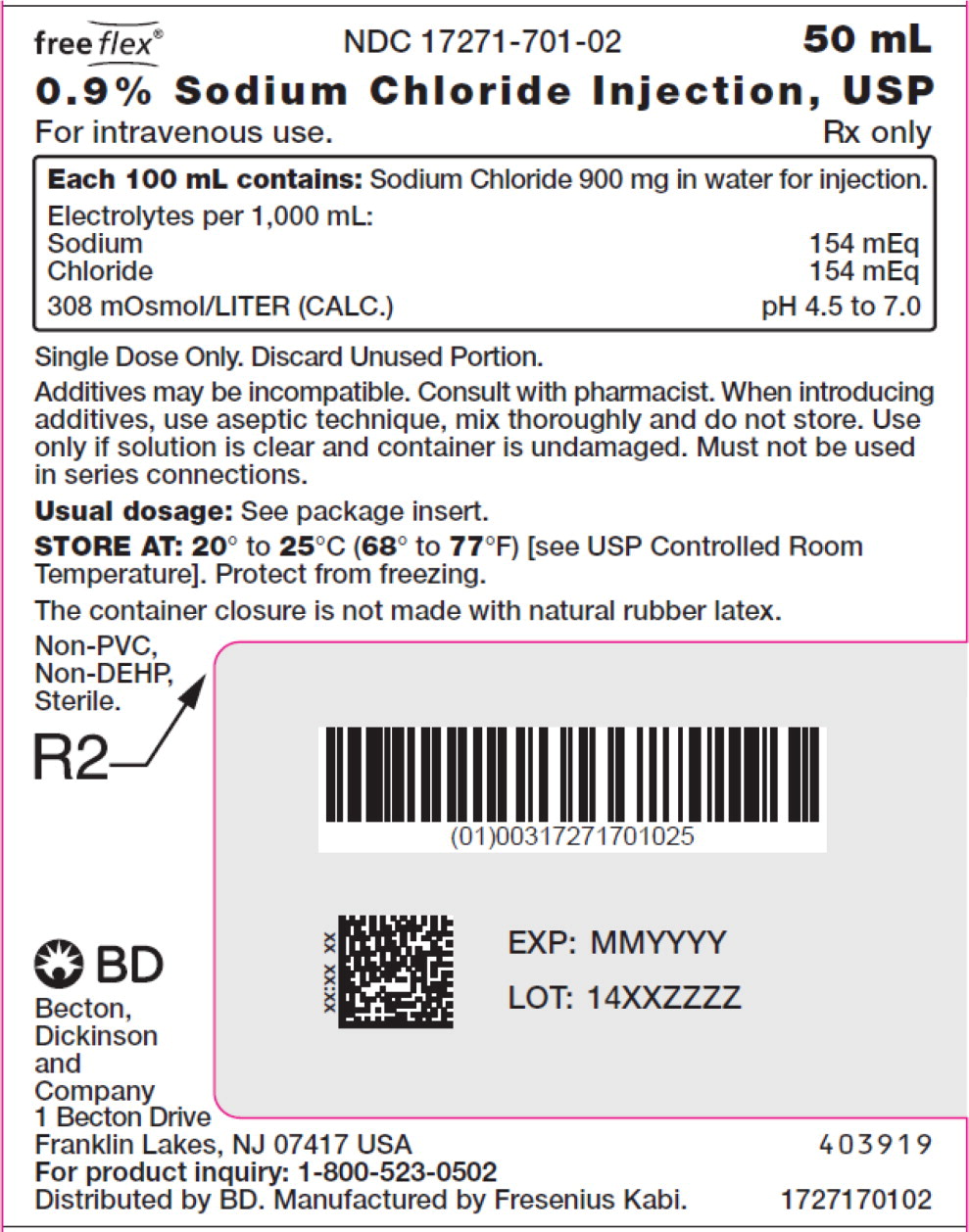
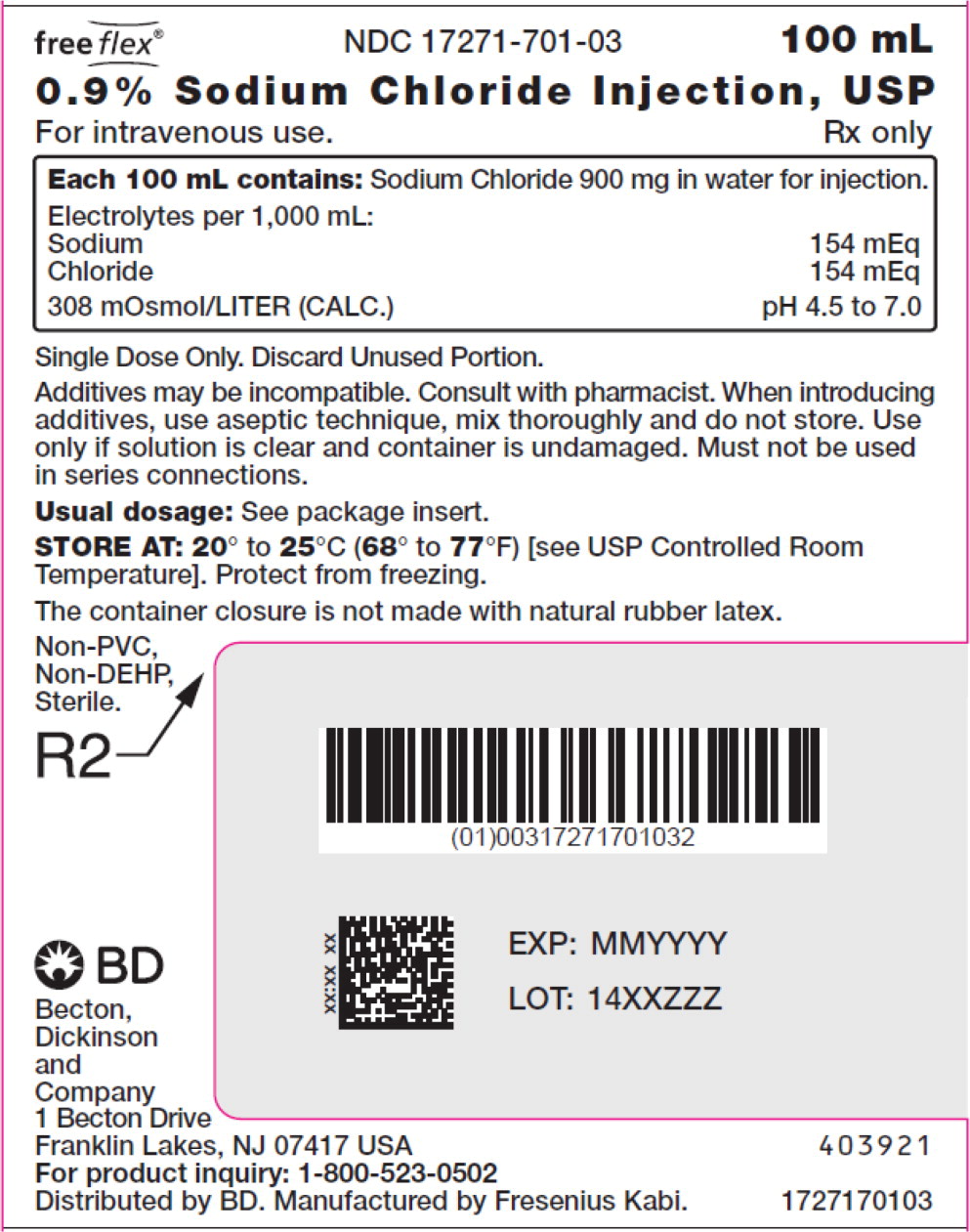
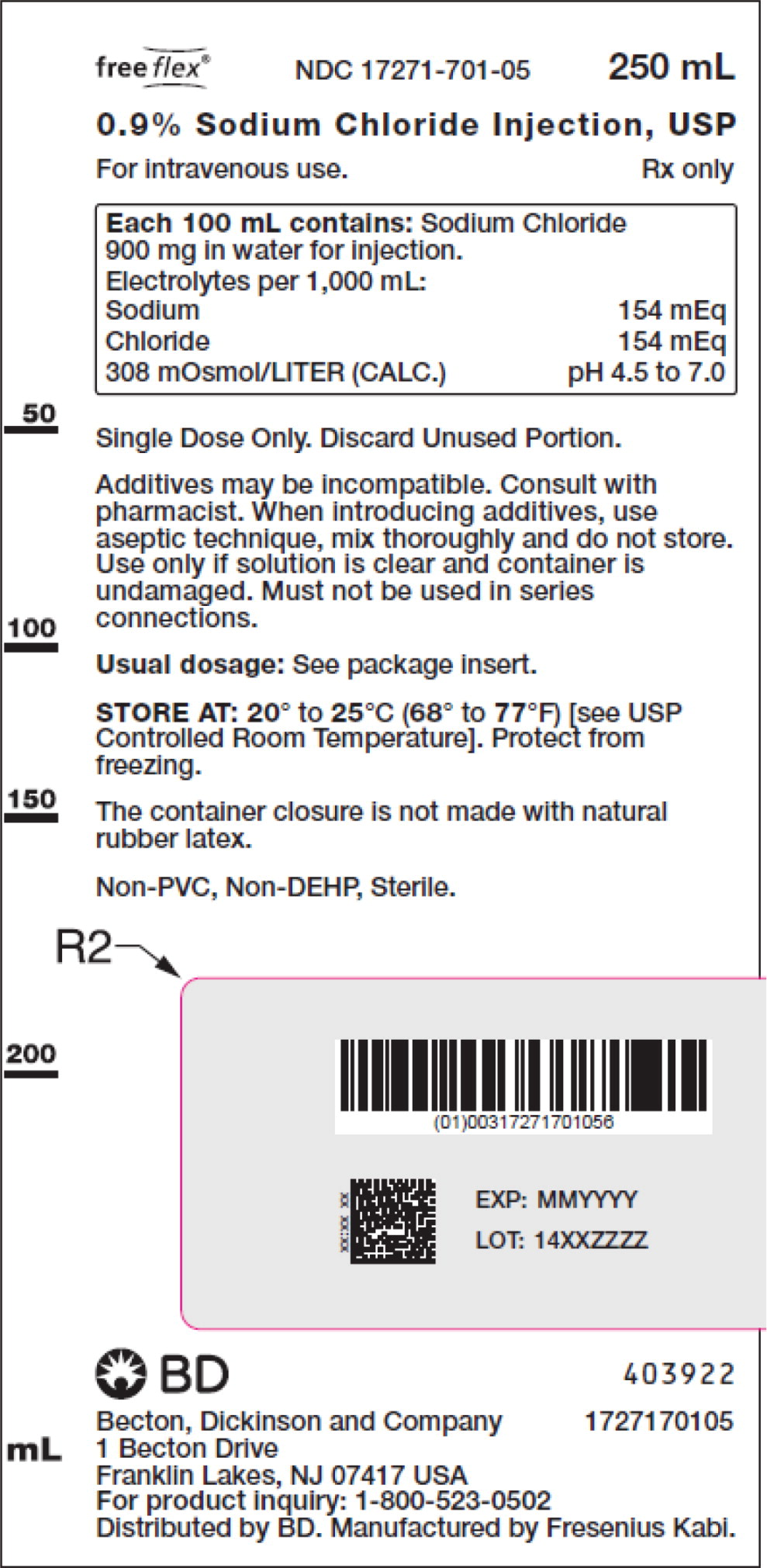
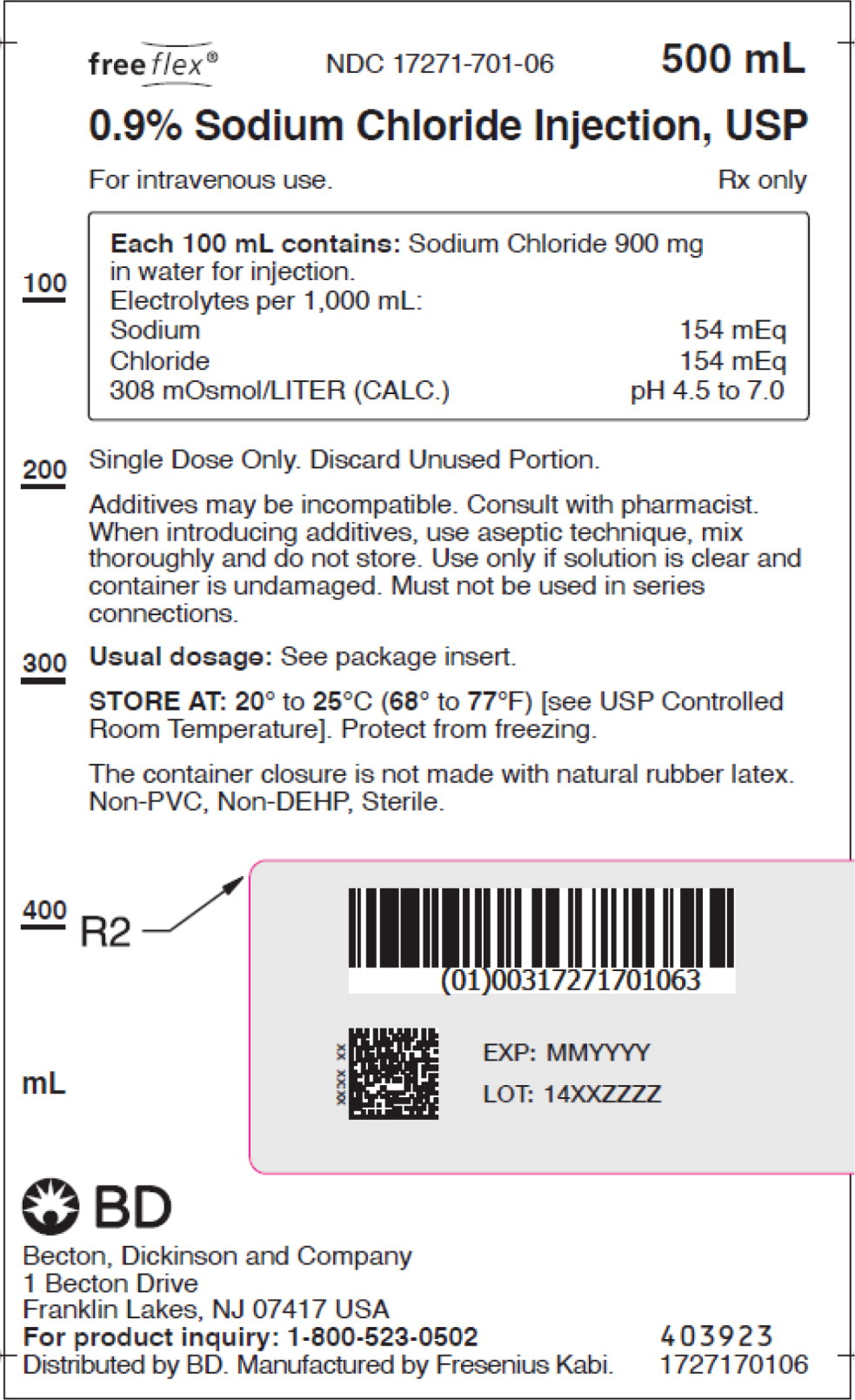
For 0.9% Sodium Chloride Injection, USP, each 100 mL contains 900 mg sodium chloride in water for injection. Electrolytes per 1,000 mL: sodium 154 mEq; chloride 154 mEq. The osmolarity is 308 mOsmol/L (calc.).
The pH in the 100 mL and smaller containers is 6.0; for the 250 mL and larger containers, the pH is 5.6.
The pH range is 4.5 to 7.0 for all containers.
The solution contains no bacteriostat, antimicrobial agent or added buffer and is intended only as a single-dose injection. When smaller doses are required the unused portion should be discarded.
The solution is a parenteral fluid and electrolyte replenisher.
Sodium Chloride, USP is chemically designated NaCl, a white crystalline powder freely soluble in water.
Water for injection, USP is chemically designated H2O.
The flexible container is fabricated from a specially formulated non-plasticized, film containing polypropylene and thermoplastic elastomers (freeflex® bag). The amount of water that can permeate from the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the flexible container can leach out certain of the container's chemical components in very small amounts within the expiration period. The suitability of the container material has been confirmed by tests in animals according to USP biological tests for plastic containers.
-
CLINICAL PHARMACOLOGY
When administered intravenously, the solution provides a source of water and electrolytes.
Solutions which provide combinations of hypotonic or isotonic concentrations of sodium chloride are suitable for parenteral maintenance or replacement of water and electrolyte requirements.
Isotonic concentrations of sodium chloride are suitable for parenteral replacement of chloride losses that exceed or equal the sodium loss. Hypotonic concentrations of sodium chloride are suited for parenteral maintenance of water requirements when only small quantities of salt are desired. A hypertonic concentration of sodium chloride may be used to repair severe salt depletion syndrome.
Sodium chloride in water dissociates to provide sodium (Na+) and chloride (Cl-) ions. Sodium (Na+) is the principal cation of the extracellular fluid and plays a large part in the therapy of fluid and electrolyte disturbances. Chloride (Cl-) has an integral role in buffering action when oxygen and carbon dioxide exchange occurs in the red blood cells. The distribution and excretion of sodium (Na+) and chloride (Cl-) are largely under the control of the kidney which maintains a balance between intake and output.
Water is an essential constituent of all body tissues and accounts for approximately 70% of total body weight. Average normal adult daily requirements range from two to three liters (1.0 to 1.5 liters each for insensible water loss by perspiration and urine production).
Water balance is maintained by various regulatory mechanisms. Water distribution depends primarily on the concentration of electrolytes in the body compartments and sodium (Na+) plays a major role in maintaining physiologic equilibrium.
- INDICATIONS AND USAGE
- CONTRAINDICATIONS
-
WARNINGS
Sodium Chloride Injection, USP should be used with great care, if at all, in patients with congestive heart failure, severe renal insufficiency and in clinical states in which there exists edema with sodium retention.
The intravenous administration of Sodium Chloride Injection, USP can cause fluid and/or solute overloading resulting in dilution of serum electrolyte concentrations, overhydration, congested states or pulmonary edema.
The risk of dilutive states is inversely proportional to the electrolyte concentration of the injections. The risk of solute overload causing congested states with peripheral and pulmonary edema is directly proportional to the electrolyte concentrations of the injections.
In patients with diminished renal function, administration of Sodium Chloride Injection, USP may result in sodium retention.
-
PRECAUTIONS
General
Do not use plastic containers in series connections. Such use could result in air embolism due to residual air being drawn from the primary container before administration of the fluid from the secondary container is completed.
Pressurizing intravenous solutions contained in flexible plastic containers to increase flow rates can result in air embolism if the residual air in the container is not fully evacuated prior to administration.
Use of a vented intravenous administration set with the vent in the open position could result in air embolism. Vented intravenous administration sets with the vent in the open position should not be used with flexible plastic containers.
-
DRUG INTERACTIONS
Caution must be exercised in the administration of Sodium Chloride Injection, USP to patients receiving corticosteroids or corticotropin.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Studies have not been performed with Sodium Chloride Injection, USP to evaluate the potential for carcinogenesis, mutagenesis or impairment of fertility.
Pregnancy:
Teratogenic Effects
Animal reproduction studies have not been conducted with Sodium Chloride Injection, USP. It is also not known whether Sodium Chloride Injection, USP can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Sodium Chloride Injection, USP should be given to a pregnant woman only if clearly needed.
Labor and Delivery
Studies have not been conducted to evaluate the effects of Sodium Chloride Injection, USP on labor and delivery. Caution should be exercised when administering this drug during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when Sodium Chloride Injection, USP is administered to a nursing mother.
Pediatric Use
The use of Sodium Chloride Injection, USP in pediatric patients is based on clinical practice.
Plasma electrolyte concentrations should be closely monitored in the pediatric population as this population may have impaired ability to regulate fluids and electrolytes.
Geriatric Use
Clinical studies of Sodium Chloride Injection, USP did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or drug therapy.
This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Do not administer unless solution is clear and container is undamaged. Discard unused portion.
-
ADVERSE REACTIONS
Reactions which may occur because of the solution or the technique of administration include febrile response, infection at the site of injection, venous thrombosis or phlebitis extending from the site of injection, extravasation and hypervolemia.
If an adverse reaction does occur, discontinue the infusion, evaluate the patient, institute appropriate therapeutic countermeasures and save the remainder of the fluid for examination if deemed necessary.
-
OVERDOSAGE
In the event of overhydration or solute overload, re-evaluate the patient and institute appropriate corrective measures (see WARNINGS, PRECAUTIONS, and ADVERSE REACTIONS).
-
DOSAGE AND ADMINISTRATION
The dose is dependent upon the age, weight and clinical condition of the patient.
Additives may be incompatible. Consult with pharmacist, if available. When introducing additives, use aseptic technique, mix thoroughly and do not store.
Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit (see PRECAUTIONS).
-
INSTRUCTIONS FOR USE
Check flexible container solution composition, lot number, and expiry date.
Do not remove solution container from its overwrap until immediately before use.
Use sterile equipment and aseptic technique.
To Open
- Turn solution container over so that the text is face down. Using the pre-cut corner tabs, peel open the overwrap and remove solution container.
- Check the solution container for leaks by squeezing firmly. If leaks are found, or if the seal is not intact, discard the solution.
- Do not use if the solution is cloudy or a precipitate is present.
To Add Medication
- Identify WHITE Additive Port with arrow pointing toward container.
- Immediately before injecting additives, break off WHITE Additive Port Cap with the arrow pointing toward container.
- Hold base of WHITE Additive Port horizontally.
- Insert needle horizontally through the center of WHITE Additive Port's septum and inject additives.
- Mix container contents thoroughly.
Preparation for Administration
- Immediately before inserting the infusion set, break off BLUE Infusion Port Cap with the arrow pointing away from container.
- Use a non-vented infusion set or close the air-inlet on a vented set.
- Close the roller clamp of the infusion set.
- Hold the base of BLUE Infusion Port.
- Insert spike through BLUE Infusion Port by rotating wrist slightly until the spike is inserted.
NOTE: See full directions accompanying administration set.
WARNING: Do not use flexible container in series connections.
-
HOW SUPPLIED
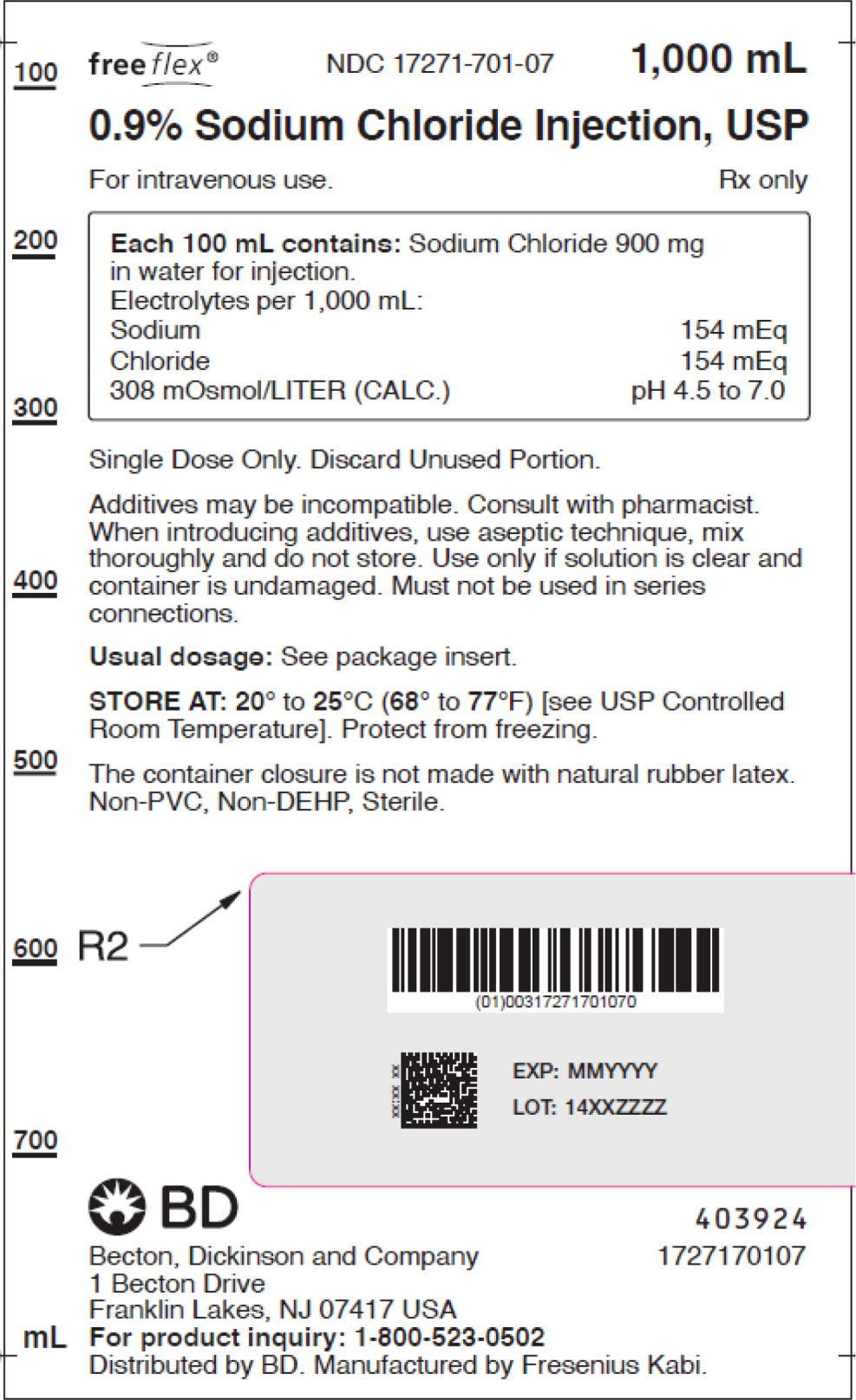
0.9% Sodium Chloride Injection, USP is supplied in single-dose flexible plastic containers as follows:
Product No. NDC
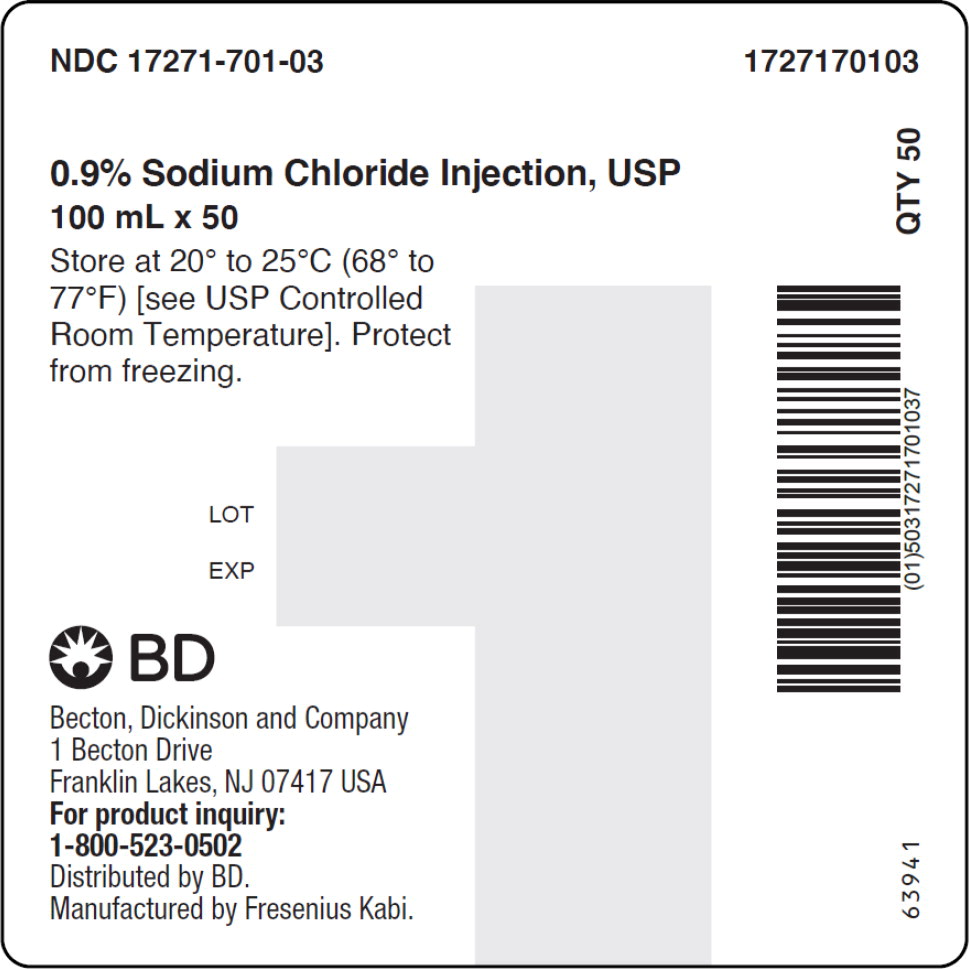
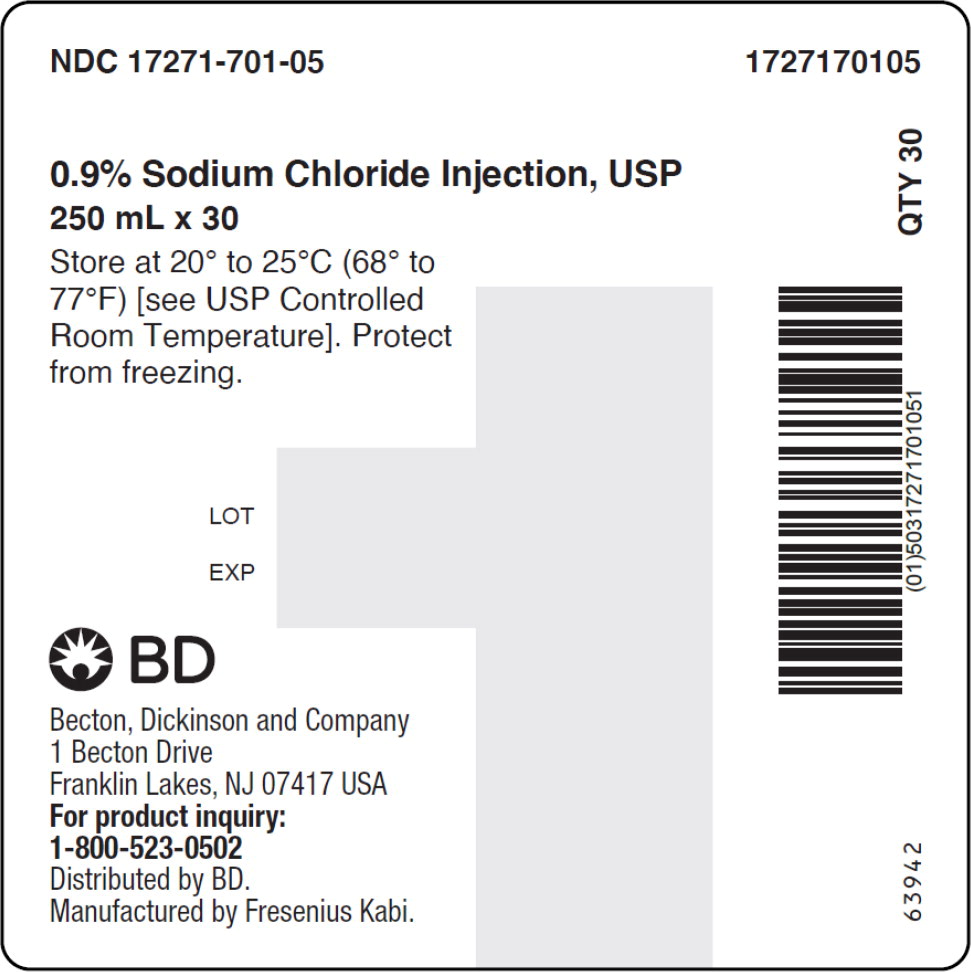
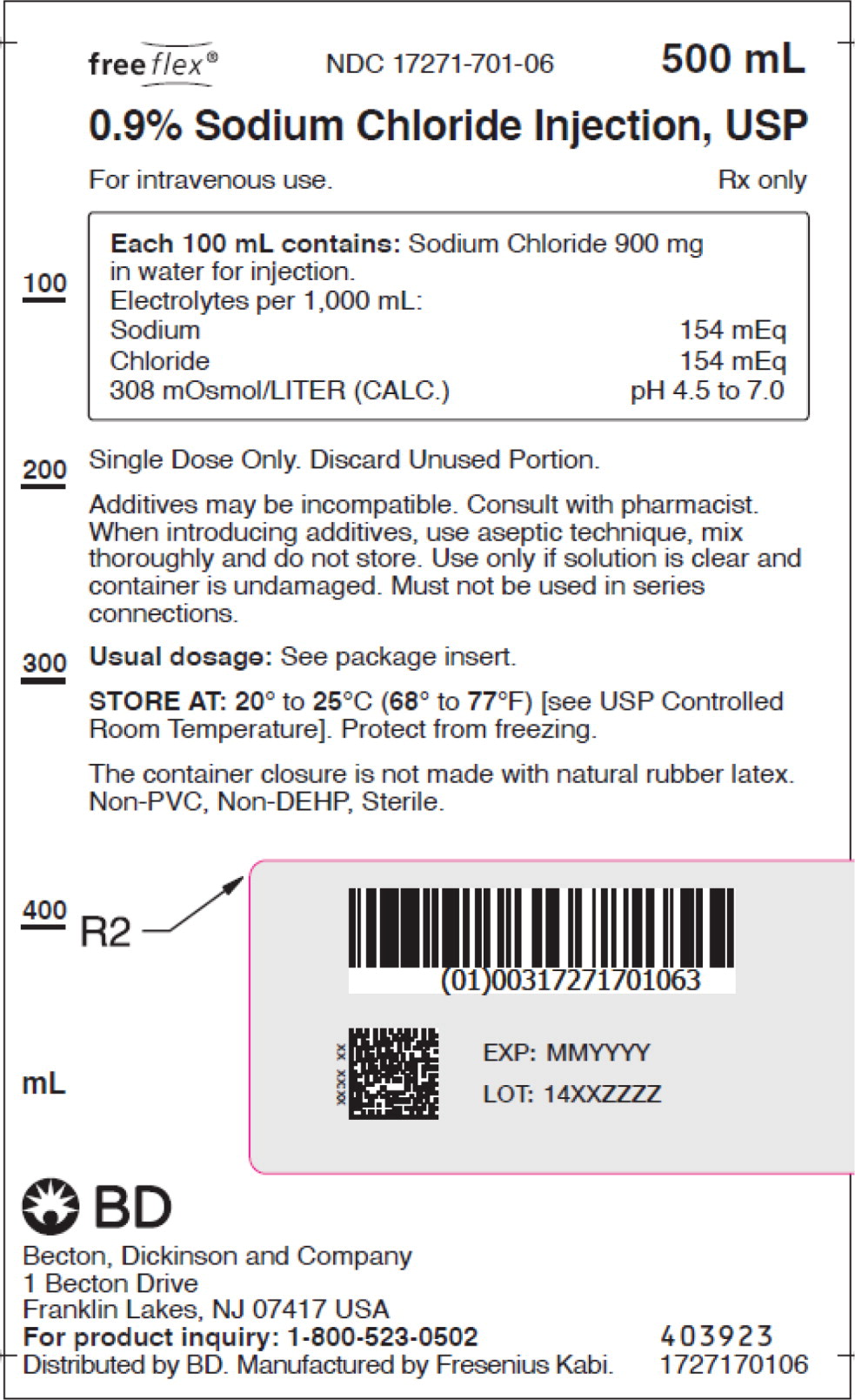
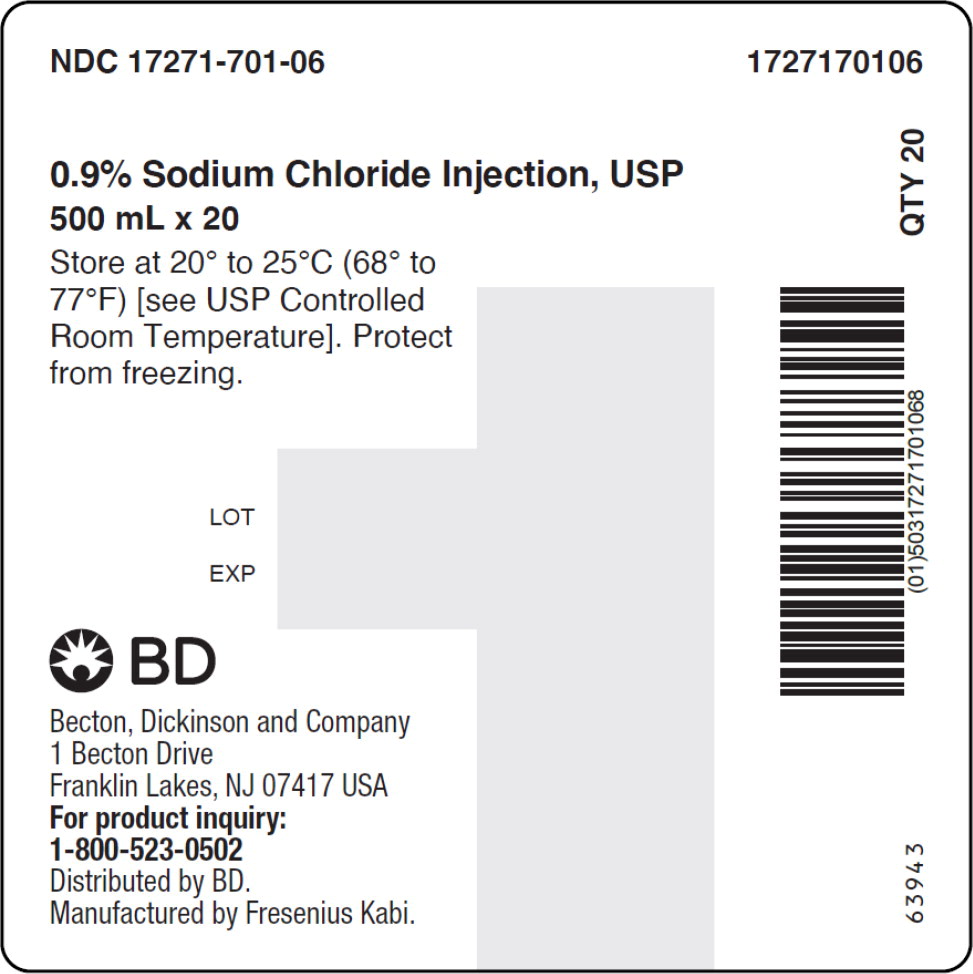
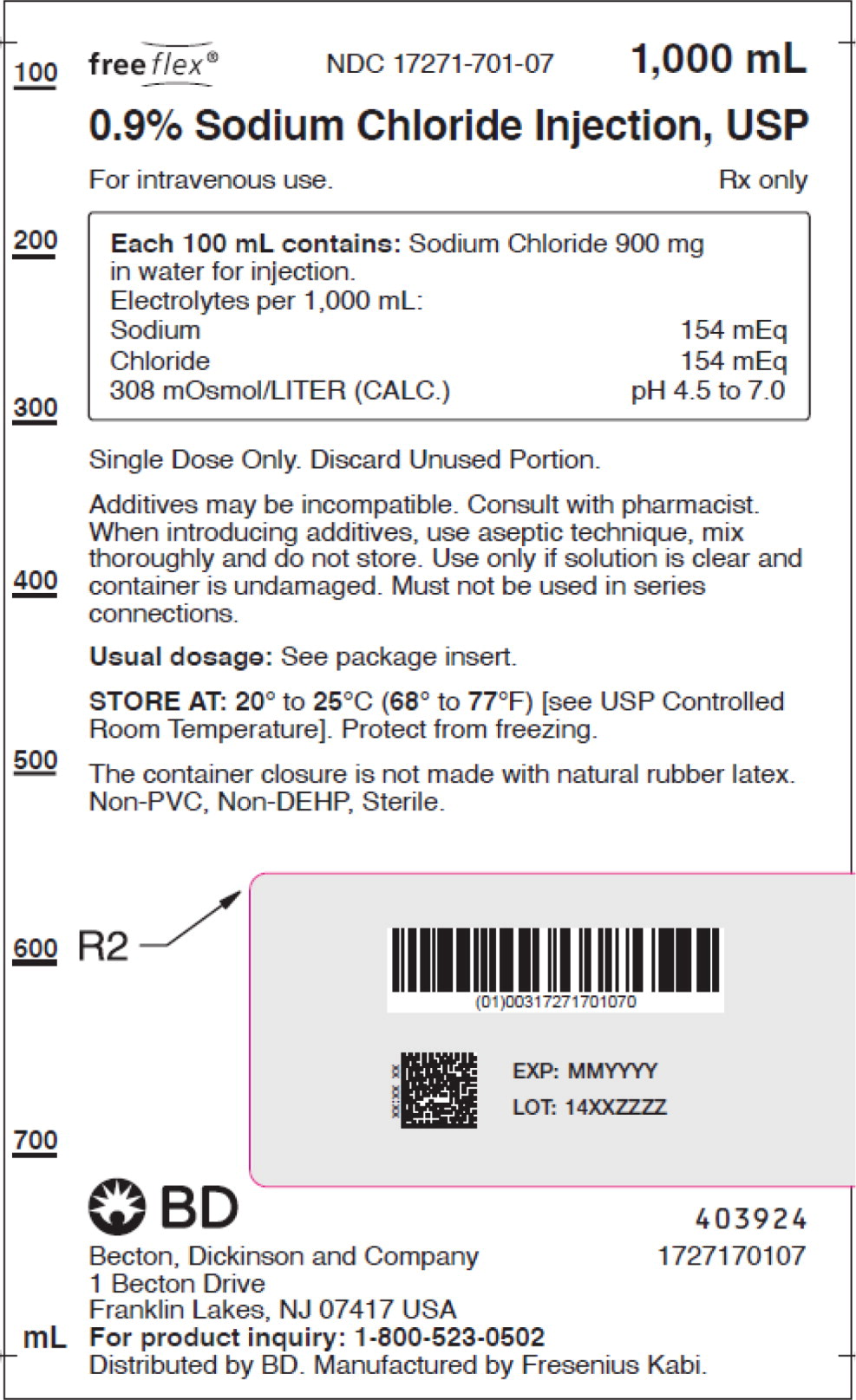
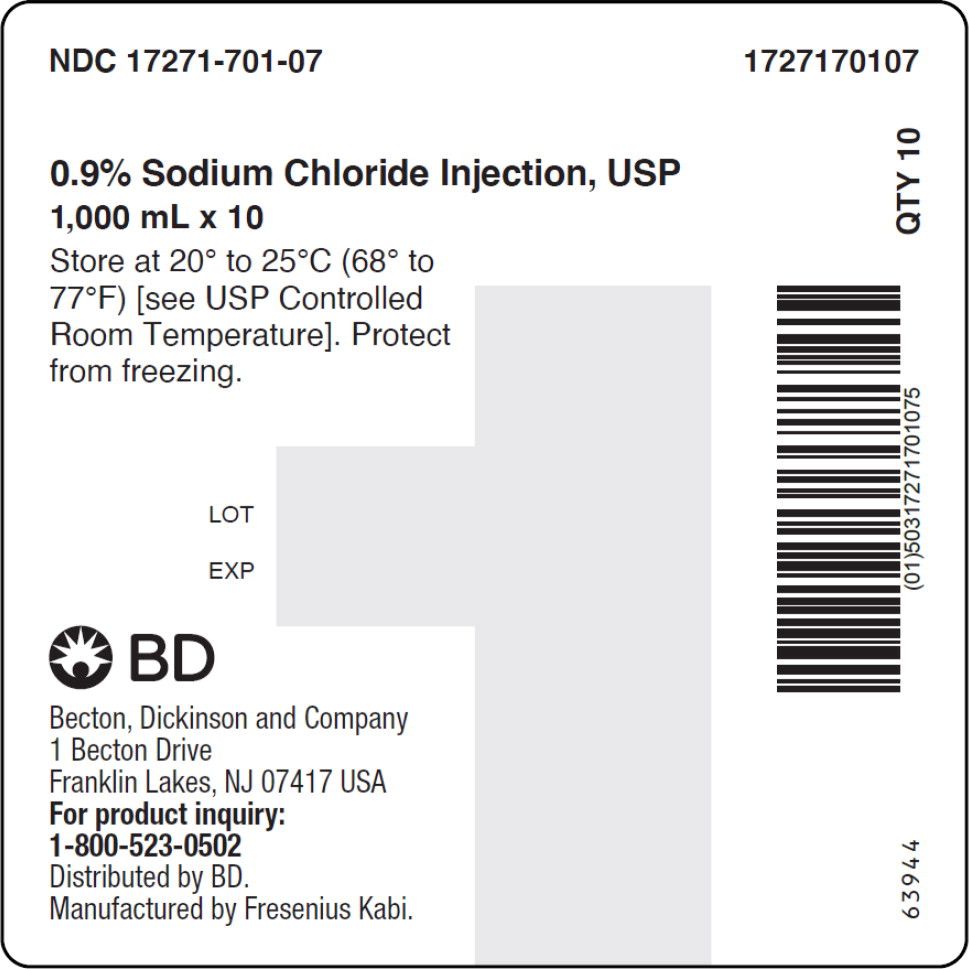
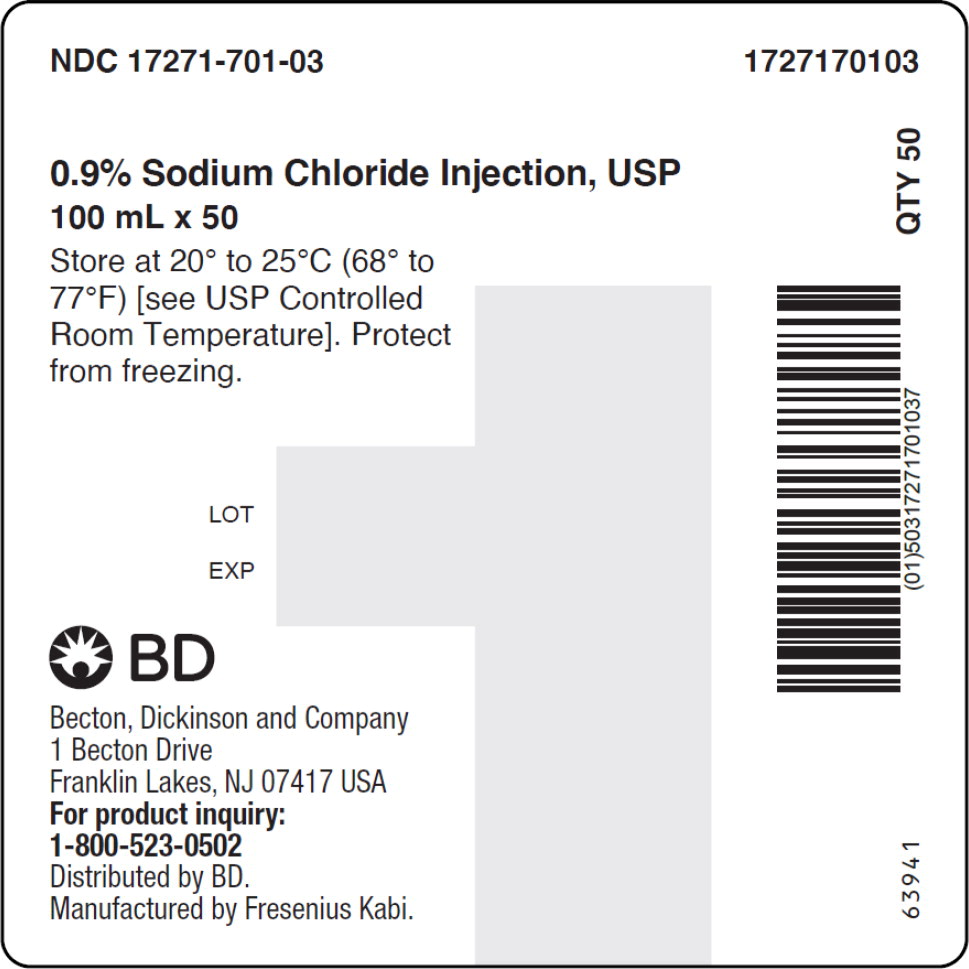
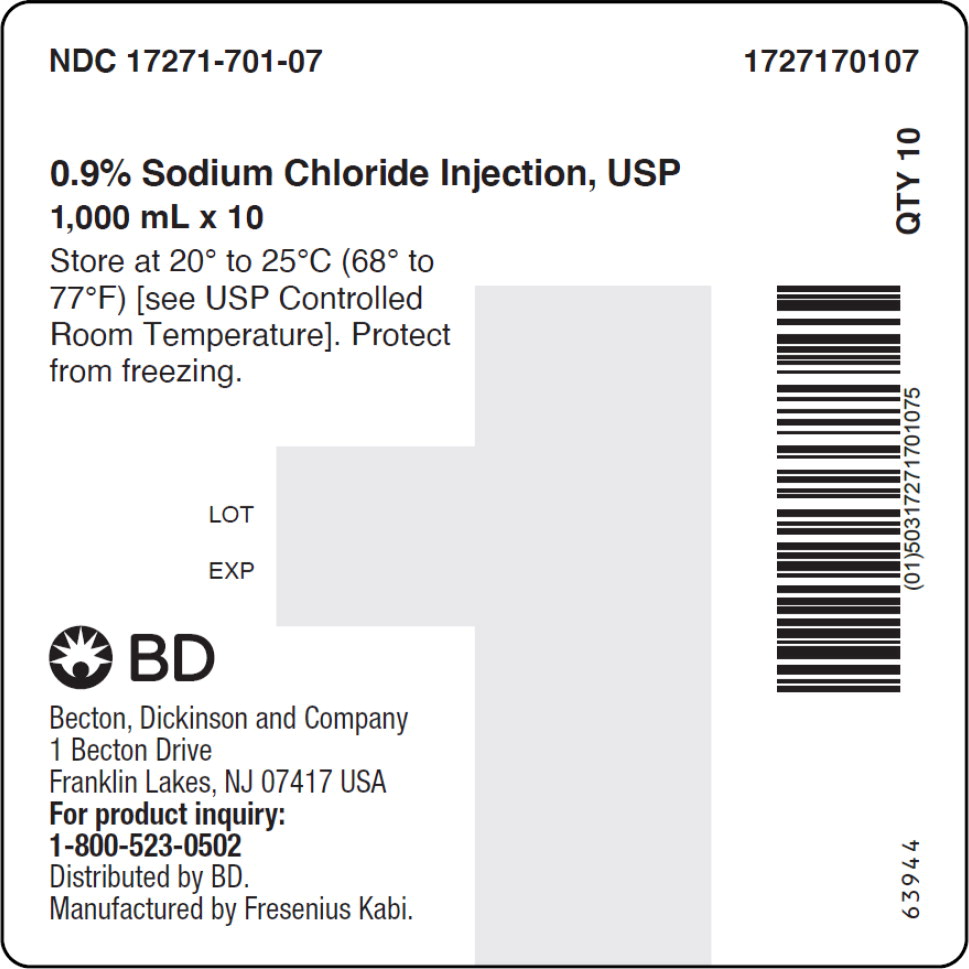
No.Strength Fill Sizes Bag Size 1727170102 17271-701-02 0.9% (9 mg/mL) 50 mL 100 mL 1727170103 17271-701-03 0.9% (9 mg/mL) 100 mL 100 mL 1727170105 17271-701-05 0.9% (9 mg/mL) 250 mL 250 mL 1727170106 17271-701-06 0.9% (9 mg/mL) 500 mL 500 mL 1727170107 17271-701-07 0.9% (9 mg/mL) 1,000 mL 1,000 mL Store at 20° to 25°C (68° to 77°F) [see USP Controlled Room Temperature]. Protect from freezing. The container closure is not made with natural rubber latex. Non-PVC, Non-DEHP, Sterile.
Becton, Dickinson and Company
1 Becton Drive
Franklin Lakes, NJ 07417 USA
For product inquiry: 1-800-523-0502
Distributed by BD. Manufactured by Fresenius Kabi.Revised: October 2022
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 50 mL Bag Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 50 mL Case Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 100 mL Bag Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 100 mL Case Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 250 mL Bag Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 250 mL Case Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 500 mL Bag Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 500 mL Case Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 1,000 mL Bag Label
- PACKAGE LABEL – PRINCIPAL DISPLAY PANEL – Sodium Chloride 1,000 mL Case Label
-
INGREDIENTS AND APPEARANCE
SODIUM CHLORIDE
sodium chloride injection, solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:17271-701 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM CHLORIDE (UNII: 451W47IQ8X) (SODIUM CATION - UNII:LYR4M0NH37, CHLORIDE ION - UNII:Q32ZN48698) SODIUM CHLORIDE 9 mg in 1 mL Inactive Ingredients Ingredient Name Strength water (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:17271-701-02 50 mL in 1 BAG; Type 0: Not a Combination Product 09/19/2017 2 NDC:17271-701-03 100 mL in 1 BAG; Type 0: Not a Combination Product 09/19/2017 3 NDC:17271-701-05 250 mL in 1 BAG; Type 0: Not a Combination Product 09/19/2017 4 NDC:17271-701-06 500 mL in 1 BAG; Type 0: Not a Combination Product 09/19/2017 5 NDC:17271-701-07 1000 mL in 1 BAG; Type 0: Not a Combination Product 09/19/2017 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA207310 09/19/2017 Labeler - Becton Dickinson and Company (124987988) Establishment Name Address ID/FEI Business Operations Fresenius Kabi Deutschland GmbH 506719546 analysis(17271-701) , manufacture(17271-701) , api manufacture(17271-701) Establishment Name Address ID/FEI Business Operations HP Halden Pharma AS 347747373 analysis(17271-701) , manufacture(17271-701) , pack(17271-701) Establishment Name Address ID/FEI Business Operations Fresenius Medical Care North America 078401205 analysis(17271-701) , label(17271-701) , manufacture(17271-701) , pack(17271-701) , sterilize(17271-701) Establishment Name Address ID/FEI Business Operations Fresenius Kabi USA, LLC 964475045 analysis(17271-701) , manufacture(17271-701)