Label: BESTMADE NATURAL PRODUCTS CALC SULPH- calc sulph tablet, soluble
- NDC Code(s): 82969-1003-1
- Packager: Bestmade Natural Products
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This homeopathic product has not been evaluated by the Food and Drug Administration for safety or efficacy. FDA is not aware of scientific evidence to support homeopathy as effective.
Drug Label Information
Updated December 17, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- PURPOSE
- DOSAGE & ADMINISTRATION
- INACTIVE INGREDIENT
- INDICATIONS & USAGE
- KEEP OUT OF REACH OF CHILDREN
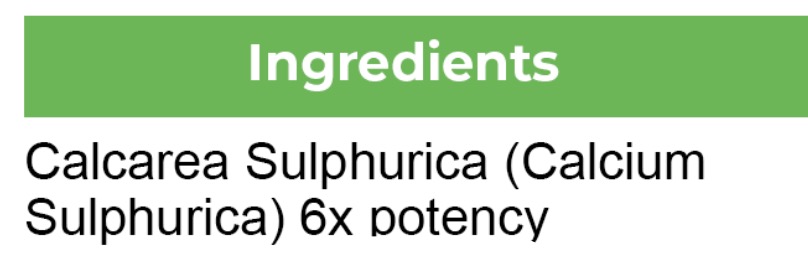
- ACTIVE INGREDIENT
- WARNINGS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
BESTMADE NATURAL PRODUCTS CALC SULPH
calc sulph tablet, solubleProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82969-1003 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength CALCIUM SULFATE (UNII: WAT0DDB505) (CALCIUM CATION - UNII:2M83C4R6ZB) CALCIUM SULFATE 6 [hp_X] in 6 [hp_X] Product Characteristics Color white (white dissolvable tablets) Score no score Shape ROUND (Round Dissolvable Tablets) Size 90mm Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82969-1003-1 6 [hp_X] in 1 BOTTLE, GLASS; Type 0: Not a Combination Product 01/01/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved homeopathic 01/01/2015 Labeler - Bestmade Natural Products (047124582) Establishment Name Address ID/FEI Business Operations Bestmade Natural Products 047124582 manufacture(82969-1003)