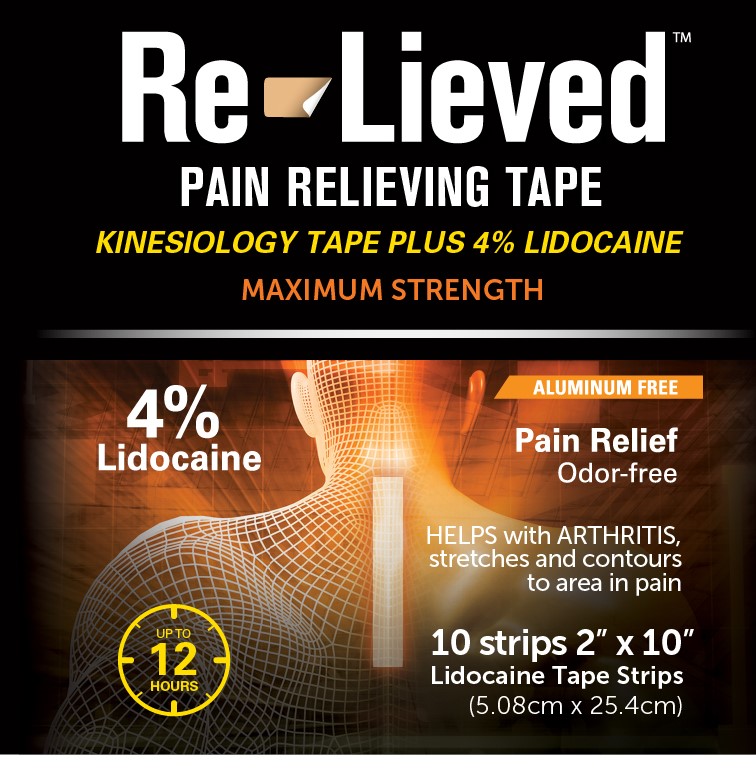
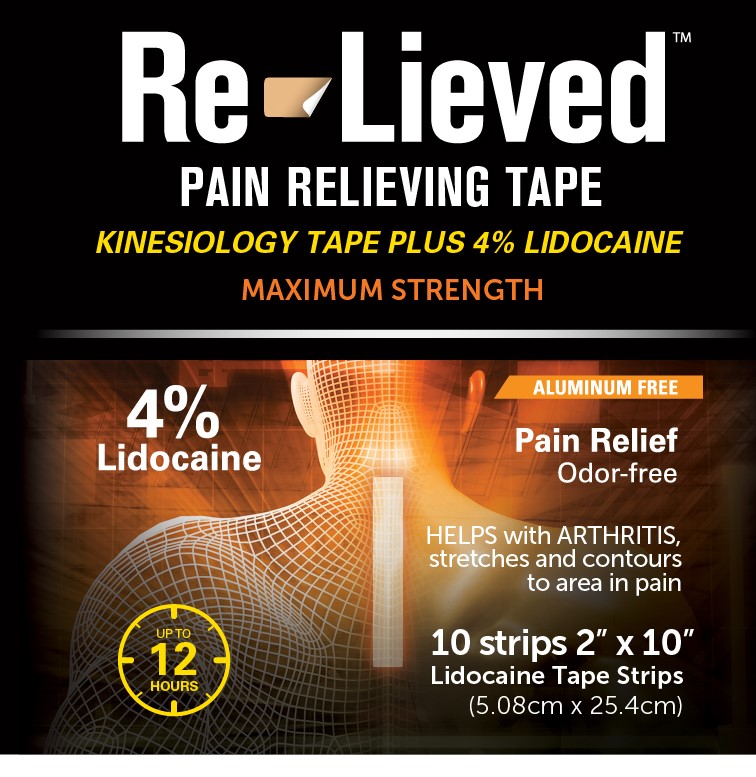
Label: RE-LIEVED 4% LIDOCAINE FABRIC TAPE- lidocaine 4% fabric tape patch
- NDC Code(s): 71662-002-10
- Packager: Transfer Technology
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated September 27, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Use
- Warnings
-
Do not use
If you are allergic to Lidocaine. On open wounds or on damaged broken or irritated skin
Remove immediately if you sense a burning sensation after application
Ask your doctor or pharmacist before use if you are using blood thining medication, steroids, or non-steroidal anti-inflammatory drugs (NSAIDS), or if you have sensitive skin.
-
WHEN USING
When using this product
- do not use other topical products
- Do not allow contact with eyes or mucous membranes
- Keep away from excessive heat. Do not apply heating pad to areea of use
- Apply only to clean, dry, and untreated skin.
- Use only as directed. Read and follow all directions and warnings on this label
- ASK DOCTOR/PHARMACIST
- PREGNANCY
- DOSAGE & ADMINISTRATION
- Other information
- Re-Lieved Pain Relieving Tape
-
INGREDIENTS AND APPEARANCE
RE-LIEVED 4% LIDOCAINE FABRIC TAPE
lidocaine 4% fabric tape patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71662-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 18 mg in 116 cm2 Inactive Ingredients Ingredient Name Strength ACRYLIC ACID/ETHYLENE COPOLYMER (600 MPA.S) (UNII: 1PEZ3NLY6I) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71662-002-10 1 in 1 PACKAGE 09/27/2022 1 1290 cm2 in 1 CYLINDER; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 09/27/2022 Labeler - Transfer Technology (037968132) Registrant - Transfer Technology (037968132) Establishment Name Address ID/FEI Business Operations Transfer Technoloy 037968132 pack(71662-002) Establishment Name Address ID/FEI Business Operations Akron Coating & Adhesives 186569323 manufacture(71662-002)