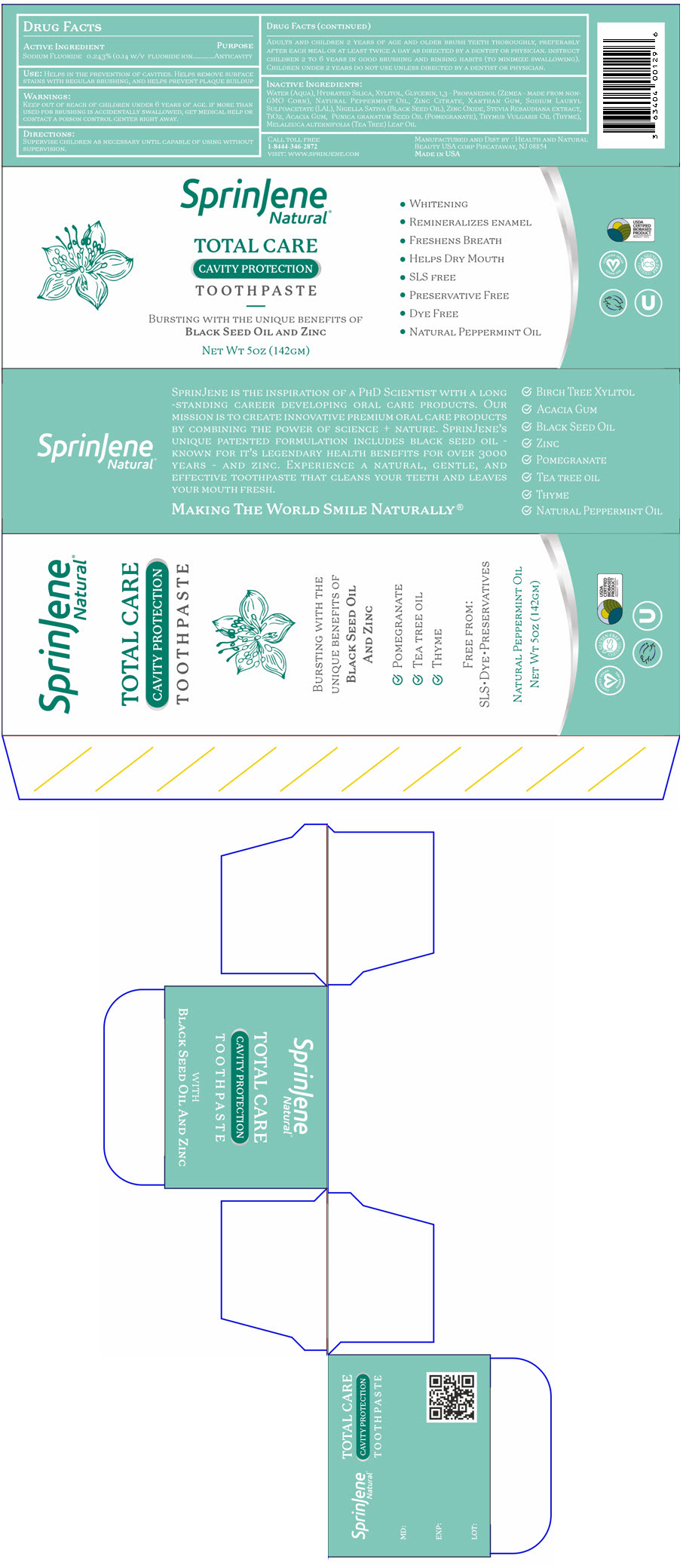
Label: SPRINJENE NATURAL TOTAL CARE CAVITY PROTECTION- sodium fluoride paste, dentifrice
- NDC Code(s): 63404-6319-1
- Packager: Health and Natural Beauty USA Corp
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 7, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
- PURPOSE
- USE
- WARNINGS
-
DIRECTIONS
SUPERVISE CHILDREN AS NECESSARY UNTIL CAPABLE OF USING WITHOUT SUPERVISION.
ADULTS AND CHILDREN 2 YEARS OF AGE AND OLDER BRUSH TEETH THOROUGHLY, PREFERABLY AFTER EACH MEAL OR AT LEAST TWICE A DAY AS DIRECTED BY A DENTIST OR PHYSICIAN. INSTRUCT CHILDREN 2 TO 6 YEARS IN GOOD BRUSHING AND RINSING HABITS (TO MINIMIZE SWALLOWING). CHILDREN UNDER 2 YEARS DO NOT USE UNLESS DIRECTED BY A DENTIST OR PHYSICIAN.
-
INACTIVE INGREDIENTS
WATER (AQUA), HYDRATED SILICA, XYLITOL, GLYCERIN, 1,3 - PROPANEDIOL (ZEMEA - MADE FROM NON-GMO CORN), NATURAL PEPPERMINT OIL, ZINC CITRATE, XANTHAN GUM, SODIUM LAURYL SULFOACETATE (LAL), NIGELLA SATIVA (BLACK SEED OIL), ZINC OXIDE, STEVIA REBAUDIANA EXTRACT, TIO2, ACACIA GUM, PUNICA GRANATUM SEED OIL (POMEGRANATE), THYMUS VULGARIS OIL (THYME), MELALEUCA ALTERNIFOLIA (TEA TREE) LEAF OIL
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 142 GM Tube Carton
SprinJene
Natural®TOTAL CARE
CAVITY PROTECTION
TOOTHPASTEBURSTING WITH THE UNIQUE BENEFITS OF
BLACK SEED OIL AND ZINCNET WT 5OZ (142GM)
- WHITENING
- REMINERALIZES ENAMEL
- FRESHENS BREATH
- HELPS DRY MOUTH
- SLS FREE
- PRESERVATIVE FREE
- DYE FREE
- NATURAL PEPPERMINT OIL
USDA
CERTIFIED
BIOBASED
PRODUCT
PRODUCT 100%CERTIFIED VEGAN
VEGAN.ORGGLUTEN-FREE
CERTIFIED
-
INGREDIENTS AND APPEARANCE
SPRINJENE NATURAL TOTAL CARE CAVITY PROTECTION
sodium fluoride paste, dentifriceProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:63404-6319 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (Fluoride Ion - UNII:Q80VPU408O) Fluoride Ion 1.5 mg in 1 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Hydrated Silica (UNII: Y6O7T4G8P9) Glycerin (UNII: PDC6A3C0OX) Xylitol (UNII: VCQ006KQ1E) PROPANEDIOL (UNII: 5965N8W85T) Xanthan Gum (UNII: TTV12P4NEE) Zinc Citrate (UNII: K72I3DEX9B) Cocamidopropyl Hydroxysultaine (UNII: 62V75NI93W) Sodium Hydroxide (UNII: 55X04QC32I) Titanium Dioxide (UNII: 15FIX9V2JP) ACACIA (UNII: 5C5403N26O) ECHINACEA PURPUREA WHOLE (UNII: QI7G114Y98) SALVIA ROSMARINUS FLOWERING TOP (UNII: 8JM482TI79) THYME OIL (UNII: 2UK410MY6B) GOLDENSEAL (UNII: ZW3Z11D0JV) Product Characteristics Color WHITE Score Shape Size Flavor PEPPERMINT Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:63404-6319-1 1 in 1 CARTON 03/01/2024 1 142 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M021 03/01/2024 Labeler - Health and Natural Beauty USA Corp (079129688) Establishment Name Address ID/FEI Business Operations Health and Natural Beauty USA Corp 079129688 MANUFACTURE(63404-6319)