Label: TRIPLE 3X MAX DIAPER RASH- zinc oxide ointment
- NDC Code(s): 16864-061-01, 16864-061-02
- Packager: Advantice Health, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient
- Purpose
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Questions?
- SPL UNCLASSIFIED SECTION
-
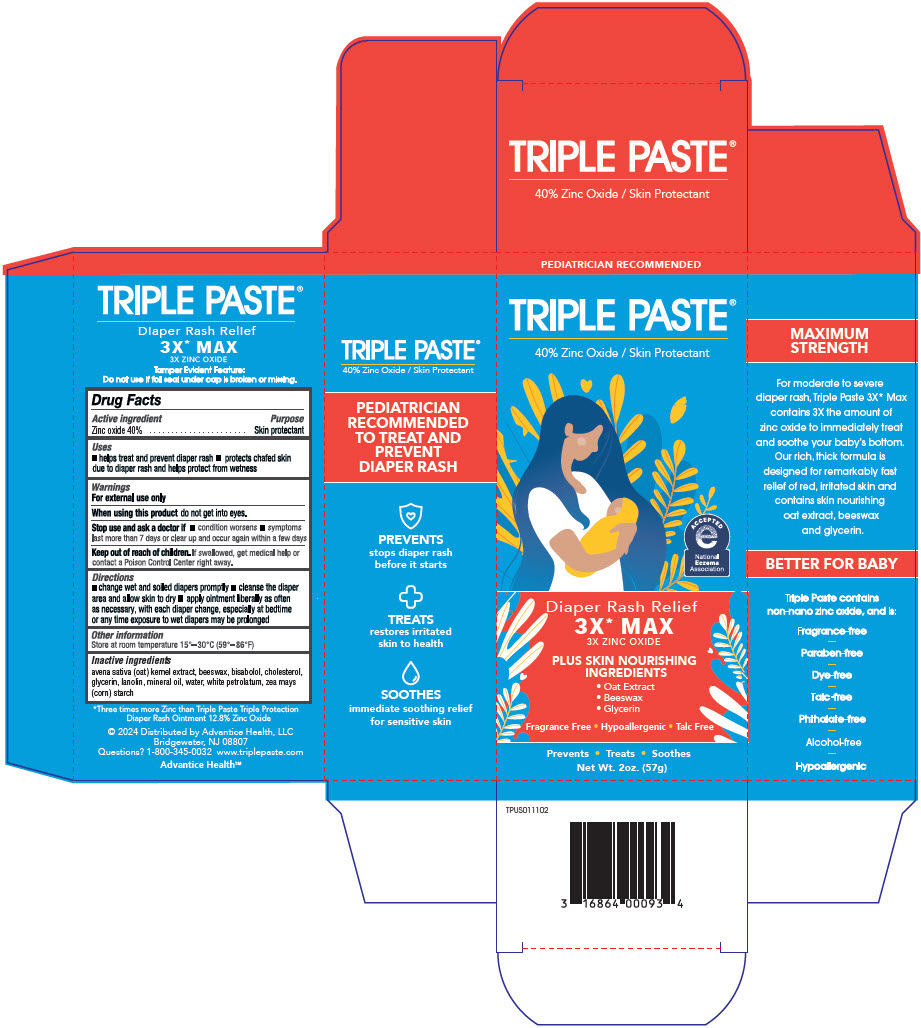
PRINCIPAL DISPLAY PANEL - 57 g Tube Carton
PEDIATRICIAN RECOMMENDED
TRIPLE PASTE ®
40% Zinc Oxide / Skin Protectant
ACCEPTED
e
National
Eczema
AssociationDiaper Rash Relief
3X* MAX
3X ZINC OXIDEPLUS SKIN NOURISHING
INGREDIENTS- Oat Extract
- Beeswax
- Glycerin
Fragrance Free • Hypoallergenic • Talc Free
Prevents • Treats • Soothes
Net Wt. 2oz. (57g)
-
INGREDIENTS AND APPEARANCE
TRIPLE 3X MAX DIAPER RASH
zinc oxide ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:16864-061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 40 g in 100 g Inactive Ingredients Ingredient Name Strength WHITE PETROLATUM (UNII: B6E5W8RQJ4) STARCH, CORN (UNII: O8232NY3SJ) LANOLIN (UNII: 7EV65EAW6H) YELLOW WAX (UNII: 2ZA36H0S2V) LEVOMENOL (UNII: 24WE03BX2T) CHOLESTEROL (UNII: 97C5T2UQ7J) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) OAT (UNII: Z6J799EAJK) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:16864-061-01 1 in 1 CARTON 05/15/2024 1 57 g in 1 TUBE; Type 0: Not a Combination Product 2 NDC:16864-061-02 170 g in 1 TUBE; Type 0: Not a Combination Product 10/15/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 05/15/2024 Labeler - Advantice Health, LLC (192527062)

