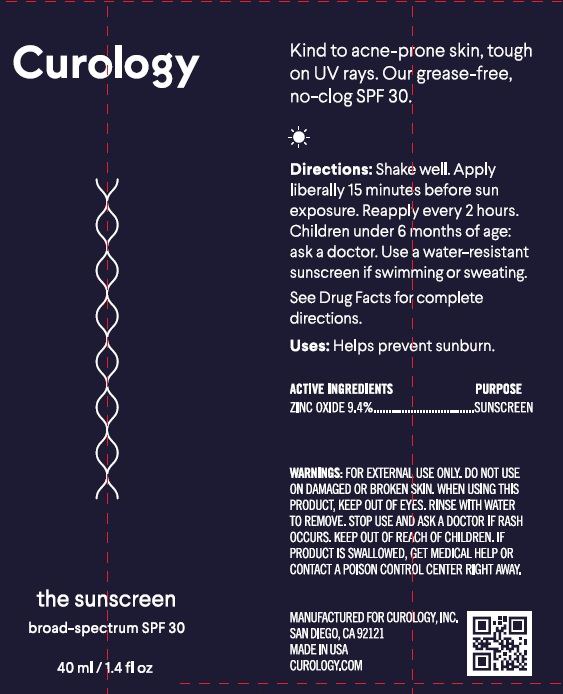
Label: CUROLOGY THE SUNSCREEN BROAD SPECTRUM SPF 30- zinc oxide cream
- NDC Code(s): 82575-032-00, 82575-032-01
- Packager: Curology Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 14, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure.
- reapply:
- immediately after towel drying
- at least every 2 hours
- Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: Sun Protection Measures.
- limit time in the sun, especially from 10 a.m.-2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses
- children under 6 months: Ask a doctor
- Other information
-
Inactive Ingredients
Water, Dimethicone, Propylene Glycol, C12-15 Alkyl Benzoate, Sorbitan Oleate Decylglucoside Crosspolymer, Cetearyl Alcohol, Argania Spinosa Kernel Oil, Ammonium Acryloyldimethyltaurate/VP Copolymer, Tocopheryl Acetate, Glycerin, Squalane, Butyrospermum Parkii (Shea Butter) Extract, Polyster-5, Caprylyl/Capryl Glucoside, Coco-Glucosdie, C14-22 Alcohols, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Triethoxycaprylylsilane, Ethyl Ferulate, C12-20 Alkyl Glucoside, Bisabolol, Sodium Chloride, Phospholipids, Gluconolactone, Benzyl Alcohol, Sodium Benzoate, Chlorophensin, Sorbic Acid, Urea, Phenoxyethanol, Ethylhexylglycerin
- Package Labeling:20ml
- Package Labeling:40ml
-
INGREDIENTS AND APPEARANCE
CUROLOGY THE SUNSCREEN BROAD SPECTRUM SPF 30
zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82575-032 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 94 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) DIMETHICONE (UNII: 92RU3N3Y1O) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ARGAN OIL (UNII: 4V59G5UW9X) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) GLYCERIN (UNII: PDC6A3C0OX) SQUALANE (UNII: GW89575KF9) SHEA BUTTER (UNII: K49155WL9Y) CAPRYLYL/CAPRYL OLIGOGLUCOSIDE (UNII: E00JL9G9K0) COCO GLUCOSIDE (UNII: ICS790225B) C14-22 ALCOHOLS (UNII: B1K89384RJ) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) ETHYL FERULATE (UNII: 5B8915UELW) C12-20 ALKYL GLUCOSIDE (UNII: K67N5Z1RUA) LEVOMENOL (UNII: 24WE03BX2T) SODIUM CHLORIDE (UNII: 451W47IQ8X) GLUCONOLACTONE (UNII: WQ29KQ9POT) BENZYL ALCOHOL (UNII: LKG8494WBH) SODIUM BENZOATE (UNII: OJ245FE5EU) CHLORPHENESIN (UNII: I670DAL4SZ) SORBIC ACID (UNII: X045WJ989B) UREA (UNII: 8W8T17847W) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82575-032-01 1 in 1 CARTON 07/16/2022 1 20 mL in 1 TUBE; Type 0: Not a Combination Product 2 NDC:82575-032-00 1 in 1 CARTON 07/16/2022 2 40 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 07/16/2022 Labeler - Curology Inc. (104103284)