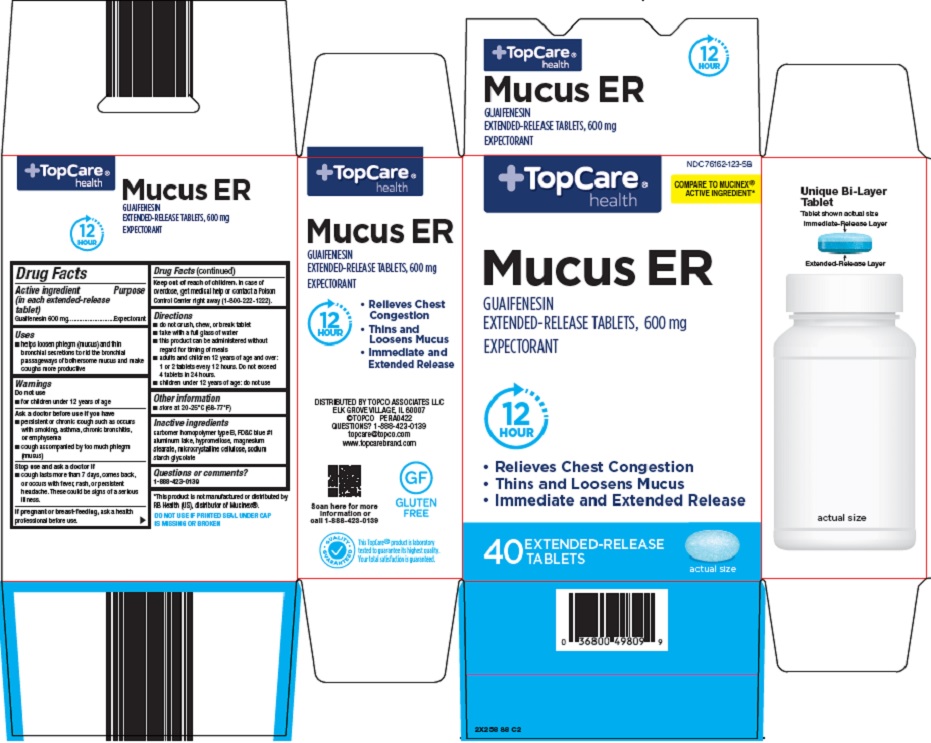
Label: TOPCARE MUCUS ER- guaifenesin tablet, multilayer, extended release
- NDC Code(s): 76162-123-58, 76162-123-60
- Packager: Topco Associates LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 12, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each extended-release tablet)
- Purpose
- Uses
-
Warnings
Ask a doctor before use if you have
- •
- persistent or chronic cough such as occurs with smoking, asthma, chronic bronchitis, or emphysema
- •
- cough accompanied by too much phlegm (mucus)
-
Directions
- •
- do not crush, chew, or break tablet
- •
- take with a full glass of water
- •
- this product can be administered without regard for the timing of meals
- •
- adults and children 12 years of age and over: 1 or 2 tablets every 12 hours. Do not exceed 4 tablets in 24 hours.
- •
- children under 12 years of age: do not use
- Other information
- Inactive ingredients
- Questions or comments?
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
TOPCARE MUCUS ER
guaifenesin tablet, multilayer, extended releaseProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76162-123 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength GUAIFENESIN (UNII: 495W7451VQ) (GUAIFENESIN - UNII:495W7451VQ) GUAIFENESIN 600 mg Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL OR ALLYL SUCROSE CROSSLINKED) (UNII: K6MOM3T5YL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) MAGNESIUM STEARATE (UNII: 70097M6I30) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) Product Characteristics Color BLUE Score no score Shape OVAL Size 16mm Flavor Imprint Code L2X2 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76162-123-58 1 in 1 CARTON 09/29/2022 1 40 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:76162-123-60 1 in 1 CARTON 11/04/2024 2 20 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date ANDA ANDA078912 09/29/2022 Labeler - Topco Associates LLC (006935977)