Label: REFRESH NATURE CREAM- citric acid, glycerin cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 82790-301-01 - Packager: GP International Corp.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 22, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
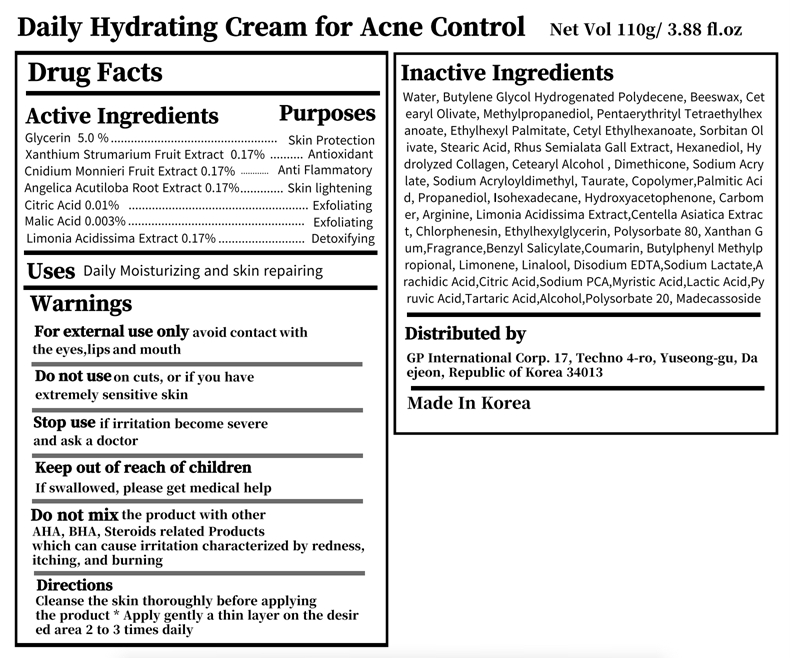
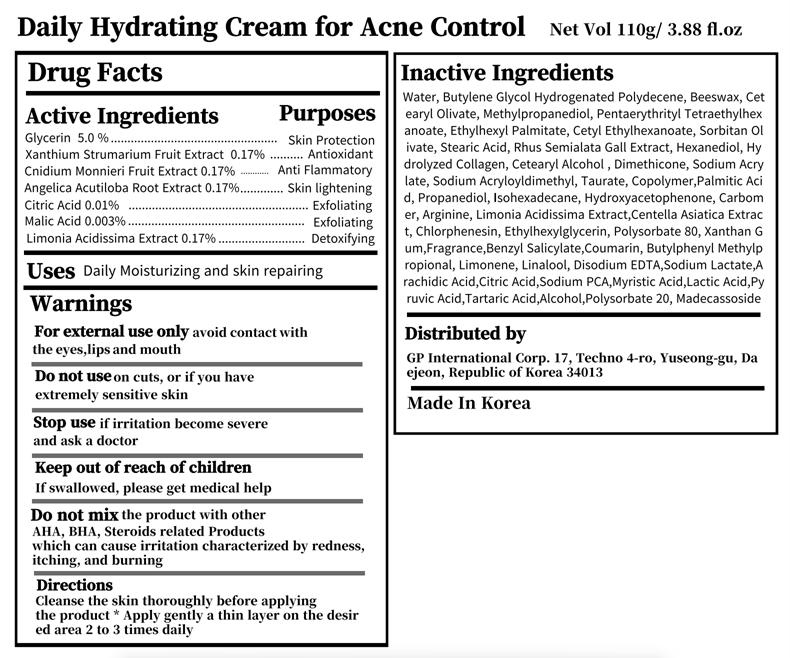
purposeAcne Control
-
UseDaily Moisturizing and skin repairing
-
WarningsDo not use on cuts, or if you have extremely sensitive skin - Do not mix the product with other AHA, BHA, Steroids related Products - which can cause irritation characterized by redness ...
-
WarningsWhen using this product do not use in or near the eyes.
-
Warnings Stop use if irritation become severe and ask a doctor
-
WarningsFor external use only avoid contact with the eyes,lips and mouth
-
WarningsKeep out of reach of children If swallowed, please get medical help
-
DirectionsDirections Cleanse the skin thoroughly before applying - the product * Apply gently a thin layer on the desired area 2 to 3 times daily
-
active ingredientGlycerin 5.0 % Skin Protection - Xanthium Strumarium Fruit Extract 0.17% Antioxidant - Cnidium Monnieri Fruit Extract 0.17% Anti Flammatory - Angelica Acutiloba Root Extract 0.17% Skin ...
-
Inactive Ingredients Water, Butylene Glycol Hydrogenated Polydecene, Beeswax, Cetearyl Olivate, Methylpropanediol, Pentaerythrityl Tetraethylhexanoate, Ethylhexyl Palmitate, Cetyl Ethylhexanoate, Sorbitan Olivate ...
-
Daily Hydrating Cream for Acne Control(What is this?)Daily Hydrating Cream for Acne Control
-
INGREDIENTS AND APPEARANCEProduct Information