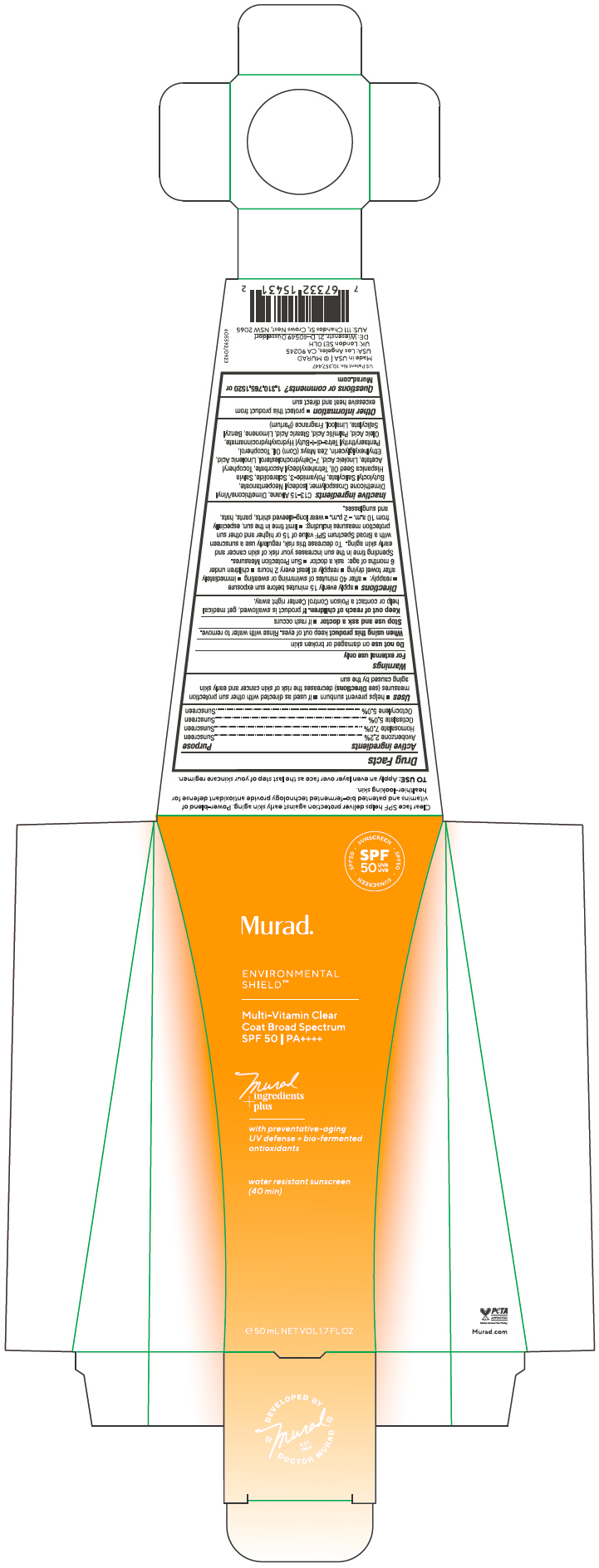
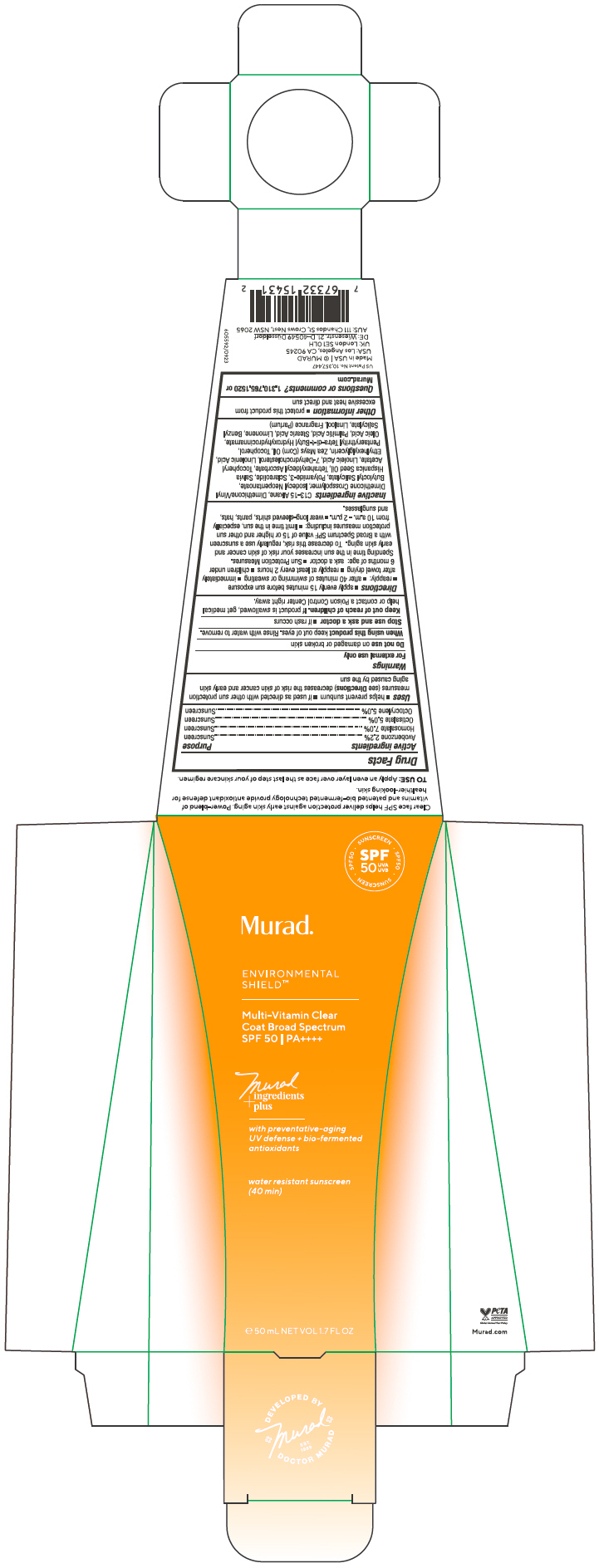
Label: MULTI VITAMIN CLEAR COAT BROAD SPECTRUM SPF 50- avobenzone, homosalate, octisalate, octocrylene gel
- NDC Code(s): 70381-125-01, 70381-125-02
- Packager: Murad, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- ACTIVE INGREDIENT
-
Uses
- helps prevent sunburn
- if used as directed with other sun protection measures (see Directions) decreases the risk of skin cancer and early skin aging caused by the sun
- Warnings
-
Directions
- apply evenly 15 minutes before sun exposure
- reapply:
- after 40 minutes of swimming or sweating
- immediately after towel drying
- reapply at least every 2 hours
- children under 6 months of age: ask a doctor
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. - 2 p.m.
- wear long-sleeved shirts, pants, hats, and sunglasses.
-
Inactive ingredients
C13-15 Alkane, Dimethicone/Vinyl Dimethicone Crosspolymer, Isodecyl Neopentanoate, Butyloctyl Salicylate, Polyamide-3, Sclareolide, Salvia Hispanica Seed Oil, Tetrahexyldecyl Ascorbate, Tocopheryl Acetate, Linoleic Acid, 7-Dehydrocholesterol, Linolenic Acid, Ethylhexylglycerin, Zea Mays (Corn) Oil, Tocopherol, Pentaerythrityl Tetra-di-t-Butyl Hydroxyhydrocinnamate, Oleic Acid, Palmitic Acid, Stearic Acid, Limonene, Benzyl Salicylate, Linalool, Fragrance (Parfum)
- Other information
- Questions or comments?
- PRINCIPAL DISPLAY PANEL - 50 mL Tube Carton
-
INGREDIENTS AND APPEARANCE
MULTI VITAMIN CLEAR COAT BROAD SPECTRUM SPF 50
avobenzone, homosalate, octisalate, octocrylene gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:70381-125 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.2 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 7 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength C13-15 ALKANE (UNII: 114P5I43UJ) DIMETHICONE/VINYL DIMETHICONE CROSSPOLYMER (SOFT PARTICLE) (UNII: 9E4CO0W6C5) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) POLYAMIDE-3 (30000 MW) (UNII: 9AO559AXEB) SCLAREOLIDE (UNII: 37W4O0O6E6) CHIA SEED OIL (UNII: MC2LH51BO7) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) LINOLEIC ACID (UNII: 9KJL21T0QJ) 7-DEHYDROCHOLESTEROL (UNII: BK1IU07GKF) LINOLENIC ACID (UNII: 0RBV727H71) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) CORN OIL (UNII: 8470G57WFM) TOCOPHEROL (UNII: R0ZB2556P8) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) OLEIC ACID (UNII: 2UMI9U37CP) PALMITIC ACID (UNII: 2V16EO95H1) STEARIC ACID (UNII: 4ELV7Z65AP) LIMONENE, (+)- (UNII: GFD7C86Q1W) BENZYL SALICYLATE (UNII: WAO5MNK9TU) LINALOOL, (+/-)- (UNII: D81QY6I88E) Product Characteristics Color YELLOW (Colorless to Light Yellow) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:70381-125-02 1 in 1 CARTON 02/15/2024 1 NDC:70381-125-01 50 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph drug M020 02/15/2024 Labeler - Murad, LLC (781254792) Establishment Name Address ID/FEI Business Operations KDC/One Chatsworth, Inc. 118542196 MANUFACTURE(70381-125)