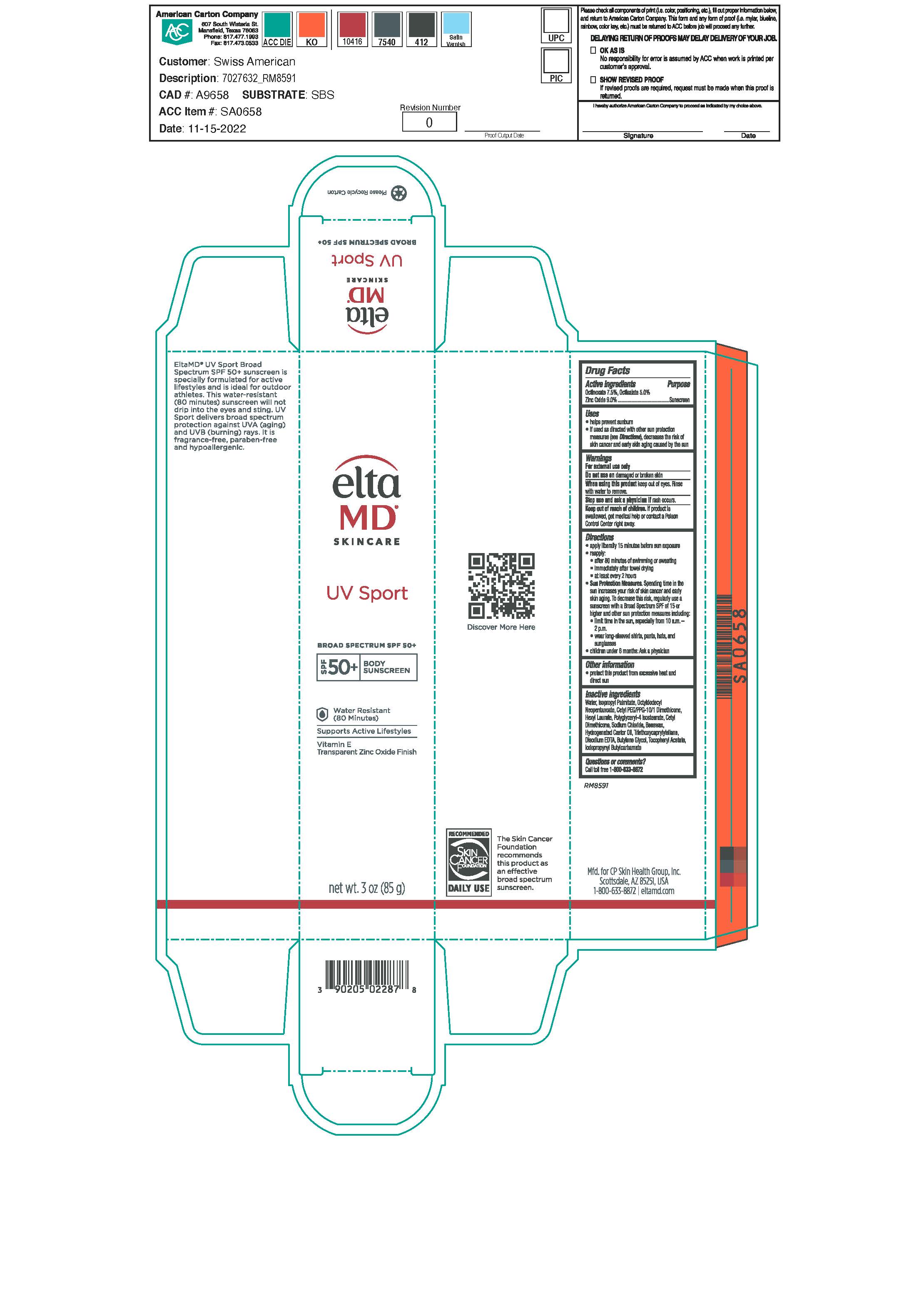
Label: ELTAMD UV SPORT SPF50- zinc oxide, octinoxate and octisalate sunscreen lotion
-
NDC Code(s):
72043-2287-2,
72043-2287-3,
72043-2287-6,
72043-2287-8, view more72043-2287-9
- Packager: CP Skin Health Group, Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 30, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Warnings
- Active ingredients
- Uses
-
Directions
Apply liberally 15 minutes before sun exposure Reapply after 80 minutes of swimming or sweating immedaitely after towel drying at least every 2 hours Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a broad spectrum SPF of 15 or higher and other sun protection measures including: limit time in the sun, especially from 10a.m.-2p.m. wear long-sleeve shirts, pants, hats and sunglasses Children under 6 months: Ask a physician
- Doseage and administration
-
Inactive ingredients
Beeswax, Butylene Glycol, Cetyl Dimethicone, Cetyl PEG/PPG-10/ 1 Dimethicone, Disodium EDTA, Hexyl Laurate, Hydrogenated Castor Oil, Iodopropynyl Butylcarbamate, Isopropyl Palmitate, Octyldoceyl Neopentaoate, Polyglyceryl-4 Isostearate, Purified Water, Sodium Chloride, Tocopheryl Acetate, Triethoxycaprylylsilane
- Keep out of reach of children
- Labeling
-
INGREDIENTS AND APPEARANCE
ELTAMD UV SPORT SPF50
zinc oxide, octinoxate and octisalate sunscreen lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:72043-2287 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 90 g in 1000 g OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE 75 g in 1000 g octisalate (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) octisalate 50 g in 1000 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) EDETATE DISODIUM (UNII: 7FLD91C86K) SODIUM CHLORIDE (UNII: 451W47IQ8X) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) IODOPROPYNYL BUTYLCARBAMATE (UNII: 603P14DHEB) ISOPROPYL PALMITATE (UNII: 8CRQ2TH63M) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) CETYL DIMETHICONE 150 (UNII: 5L694Y0T22) YELLOW WAX (UNII: 2ZA36H0S2V) HYDROGENATED CASTOR OIL (UNII: ZF94AP8MEY) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) .ALPHA.-TOCOPHEROL ACETATE, DL- (UNII: WR1WPI7EW8) CETYL PEG/PPG-10/1 DIMETHICONE (HLB 1.5) (UNII: V2W71V8T0X) POLYGLYCERYL-4 ISOSTEARATE (UNII: 820DPX33S7) HEXYL LAURATE (UNII: 4CG9F9W01Q) Product Characteristics Color white Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:72043-2287-3 84 g in 1 TUBE; Type 0: Not a Combination Product 01/10/2018 2 NDC:72043-2287-9 226 g in 1 TUBE; Type 0: Not a Combination Product 05/30/2024 3 NDC:72043-2287-8 198 g in 1 BOTTLE; Type 0: Not a Combination Product 01/10/2018 05/30/2024 4 NDC:72043-2287-2 2 g in 1 PACKET; Type 0: Not a Combination Product 07/07/2022 5 NDC:72043-2287-6 1815 g in 1 BOTTLE; Type 0: Not a Combination Product 07/07/2022 09/11/2023 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 01/10/2018 Labeler - CP Skin Health Group, Inc. (611921669) Registrant - Swiss-American CDMO, LLC (080170933) Establishment Name Address ID/FEI Business Operations Swiss-American CDMO, LLC 080170933 manufacture(72043-2287)