Label: HAND SANITIZER gel
- NDC Code(s): 65692-2073-2
- Packager: Raining Rose
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 8, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- PURPOSE
- INDICATIONS & USAGE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DOSAGE & ADMINISTRATION
- OTHER SAFETY INFORMATION
- INACTIVE INGREDIENT
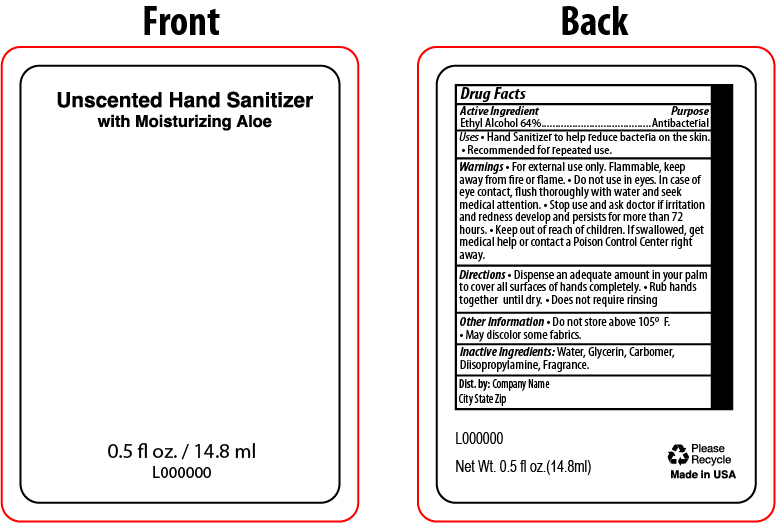
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HAND SANITIZER
hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:65692-2073 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 64 mL in 100 mL Inactive Ingredients Ingredient Name Strength CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) WATER (UNII: 059QF0KO0R) DIISOPROPYLAMINE (UNII: BR9JLI40NO) GLYCERIN (UNII: PDC6A3C0OX) Product Characteristics Color Score Shape Size Flavor CITRUS Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:65692-2073-2 14.8 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/10/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/10/2022 Labeler - Raining Rose (083819404) Registrant - Raining Rose (083819404) Establishment Name Address ID/FEI Business Operations Chemisphere 071957146 manufacture(65692-2073) Establishment Name Address ID/FEI Business Operations Raining Rose 083819404 label(65692-2073)