Label: KILL FIRETOOTHPASTE- sodium fluoride paste
- NDC Code(s): 24765-133-01
- Packager: Pharmacal-International. Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated August 17, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
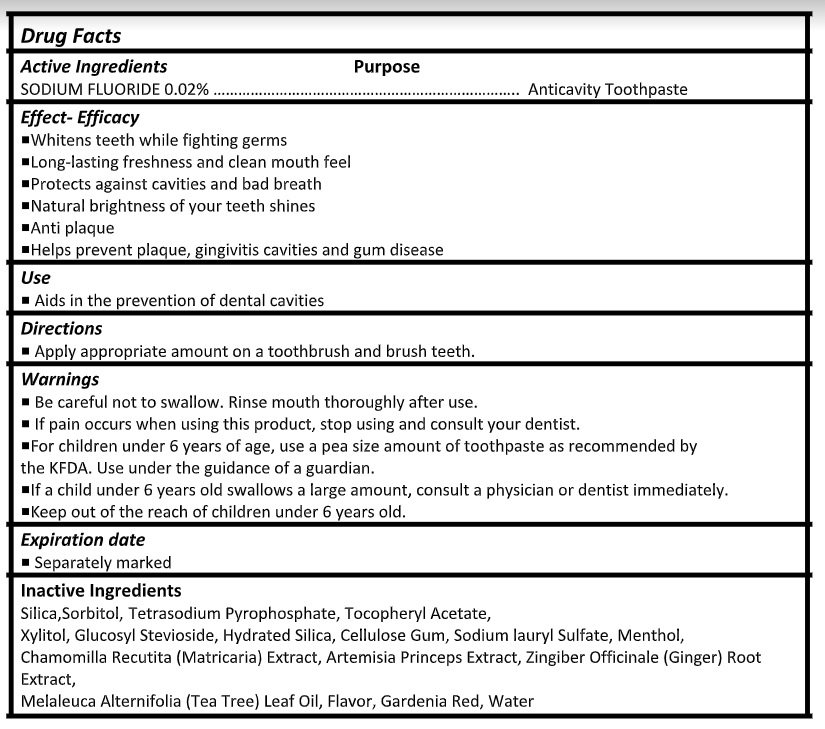
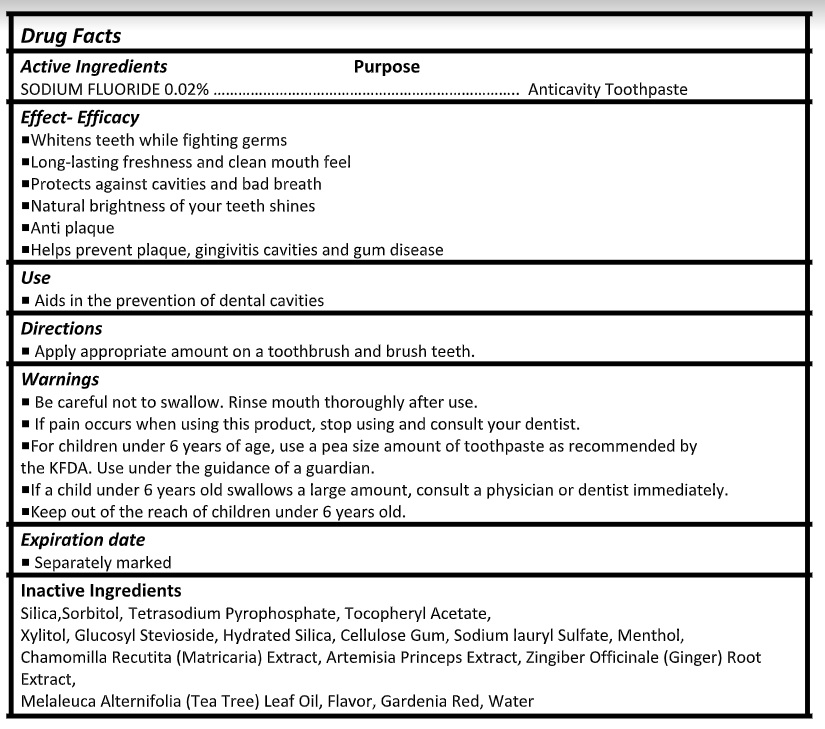
Active ingredient
Sodium Fluoride 0.02%
Warnings
- Be careful not to swallow. Rinse mouth thoroughly after use.
- If pain occurs when using this product, stop using and consult your dentist.
- For children under 6 years of age, use a pea size amount of toothpaste as recommended by the KFDA. Use under the guidance of a guardian.
- If a child under 6 years old swallows a large amount, consult a physician or dentist immediately.
- Keep out of the reach of children under 6 years old.
Inactive ingredients
Silica,Sorbitol, Tetrasodium Pyrophosphate, Tocopheryl Acetate,
Xylitol, Glucosyl Stevioside, Hydrated Silica, Cellulose Gum, Sodium lauryl Sulfate, Menthol,
Chamomilla Recutita (Matricaria) Extract, Artemisia Princeps Extract, Zingiber Officinale (Ginger) Root Extract,
Melaleuca Alternifolia (Tea Tree) Leaf Oil, Flavor, Gardenia Red, Water - Product label
-
INGREDIENTS AND APPEARANCE
KILL FIRETOOTHPASTE
sodium fluoride pasteProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:24765-133 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.02 g in 100 g Inactive Ingredients Ingredient Name Strength SODIUM PYROPHOSPHATE (UNII: O352864B8Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) SORBITOL (UNII: 506T60A25R) XYLITOL (UNII: VCQ006KQ1E) HYDRATED SILICA (UNII: Y6O7T4G8P9) SODIUM LAURYL SULFATE (UNII: 368GB5141J) MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) MATRICARIA CHAMOMILLA WHOLE (UNII: G0R4UBI2ZZ) GINGER (UNII: C5529G5JPQ) TEA TREE OIL (UNII: VIF565UC2G) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:24765-133-01 100 g in 1 TUBE; Type 0: Not a Combination Product 06/15/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 06/15/2021 Labeler - Pharmacal-International. Co., Ltd. (557805060) Registrant - Pharmacal-International. Co., Ltd. (557805060) Establishment Name Address ID/FEI Business Operations KMPHARMACEUTICAL Co.,Ltd. 689850153 manufacture(24765-133)