Label: METHYL SALICYLATE 25 PERCENT- methyl salicylate cream
- NDC Code(s): 50488-1016-5
- Packager: Alexso, Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated May 23, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
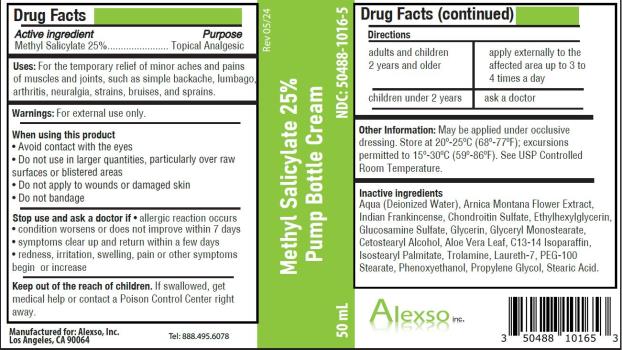
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only.
When using this product
- Avoid contact with the eyes
- Do not use in large quantities, particularly over raw surfaces or blistered areas
- Do not apply to wounds or damaged skin
- Do not bandage
- Avoid contact with the eyes
- Directions
- Other information
-
Inactive ingredients
Aqua (Deionized Water), Arnica Montana Flower Extract, Indian Frankincense, Chondroitin Sulfate, Ethylhexylglycerin, Glucosamine Sulfate, Glycerin, Glyceryl Monostearate, Cetostearyl Alcohol, Aloe Vera Leaf, C13-14 Isoparaffin, Isostearyl Palmitate, Trolamine, Laureth-7, PEG-100 Stearate, Phenoxyethanol, Propylene Glycol, Stearic Acid.
Methyl Salicylate 25% Pump Bottle Cream
NDC: 50488-1016-5
50 mL
Manufactured for:
Alexso, Inc
Los Angeles, CA 90064Tel: 888.495.6078
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
METHYL SALICYLATE 25 PERCENT
methyl salicylate creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50488-1016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength METHYL SALICYLATE (UNII: LAV5U5022Y) (SALICYLIC ACID - UNII:O414PZ4LPZ) METHYL SALICYLATE 250 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) ARNICA MONTANA FLOWER (UNII: OZ0E5Y15PZ) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) CHONDROITIN SULFATE (BOVINE) (UNII: 6IC1M3OG5Z) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) GLUCOSAMINE SULFATE (UNII: 1FW7WLR731) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) ALOE VERA LEAF (UNII: ZY81Z83H0X) C13-14 ISOPARAFFIN (UNII: E4F12ROE70) ISOSTEARYL PALMITATE (UNII: 9EHU0R7ER1) TROLAMINE (UNII: 9O3K93S3TK) LAURETH-7 (UNII: Z95S6G8201) PEG-100 STEARATE (UNII: YD01N1999R) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) STEARIC ACID (UNII: 4ELV7Z65AP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50488-1016-5 50 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 05/01/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 05/01/2024 Labeler - Alexso, Inc (963338061)