Label: CVS SORE THROAT MIXED BERRY FLAVOR- benzocaine, dextromethorphan hydrobromide lozenge
-
Contains inactivated NDC Code(s)
NDC Code(s): 59779-649-18 - Packager: CVS Pharmacy, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated April 22, 2014
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- CVS Sore Throat Mixed Berry Flavor
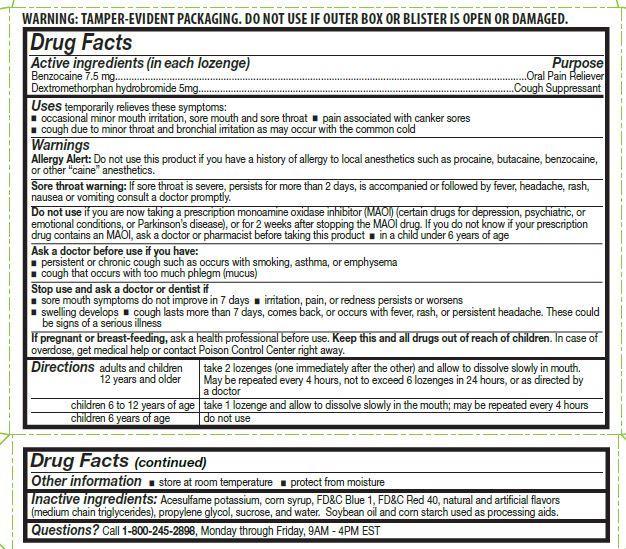
- ACTIVE INGREDIENT
- Uses
-
Warnings
Allergy Alert: Do not use this product if you have a history of allergy to local anesthetics such as procaine, butacaine, benzocaine, or any other "caine" anesthetics.
Sore throat warning:
Severe or persistent sore throat or sore throat accompanied by high fever, headache, rash, nausea, and vomiting may be serious. Consult a dentist or doctor right away. Do not use more than 2 days or administer to children under 5 years of age unless directed by a doctor.
Do not use if you are now taking a prescription monoamine oxidase inhibitor (MAOI) (certain drugs for depression, psychiatric, or emotional conditions, or Parkinson’s disease), or for 2 weeks after stopping the MAOI drug. If you do not know if your prescription drug contains an MAOI, ask a doctor or pharmacist before taking this product.
Stop Use and ask a doctor or dentist if
- sore mouth symptom do not improve in 7 days
- irritation, pain or redness persists or worsens
- swelling developes
- cough lasts for more than 7 days, comes back, or is accompanied by fever, rash, or persistent headache. A persistent cough may be a sign of a serious condition.
-
Directions
-
adults and children 12 years and older: take 2 lozenges (one immediately after the other) and allow to dissolve slowly in the mouth. May be repeated every 4 hours, not to exceed 6 lozenges in 24-hours, or as directed by a doctor
-
children 6 to under 12 years of age: take 1 lozenge and allow to dissolve slowly in the mouth; may be repeated every 4 hours
-
children 6 years of age: do not use
-
- Other information
- Inactive ingredients
- Questions ?
-
CVS Sore Throat Lozenges Mixed Berry Flavor 18 Count (59779-649-18)
CVS pharmacy
compare to the active ingredients in Cepacol**
SORE THROAT &
COUGH LOZENGES
SOOTHING RELIEF
BENZOCAINE 7.5 mg/DEXTROMETHORPHAN HBr 5 mg
Temporarily relieves:
- Minor sore throat &mouth irritation
- Cough due to minor throat irritation
Mixed Berry Flavor
18 LOZENGES
DISTRIBUTED BY:
CVS Pharmacy, Inc.
One Cvs Drive Woonsocket, RI 02895
2014 CVS/Pharmacy
CVS.com 1-800-SHOP CVS
Made in the USA
This product is not manufactured or distributed
by Reckftt Bencldser owner af the registered
trademark Cepacol.


-
INGREDIENTS AND APPEARANCE
CVS SORE THROAT MIXED BERRY FLAVOR
benzocaine, dextromethorphan hydrobromide lozengeProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-649 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZOCAINE (UNII: U3RSY48JW5) (BENZOCAINE - UNII:U3RSY48JW5) BENZOCAINE 7.5 mg DEXTROMETHORPHAN HYDROBROMIDE (UNII: 9D2RTI9KYH) (DEXTROMETHORPHAN - UNII:7355X3ROTS) DEXTROMETHORPHAN HYDROBROMIDE 5 mg Inactive Ingredients Ingredient Name Strength ACESULFAME POTASSIUM (UNII: 23OV73Q5G9) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) CORN SYRUP (UNII: 9G5L16BK6N) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SUCROSE (UNII: C151H8M554) WATER (UNII: 059QF0KO0R) SOYBEAN OIL (UNII: 241ATL177A) STARCH, CORN (UNII: O8232NY3SJ) Product Characteristics Color purple Score no score Shape ROUND Size 23mm Flavor BERRY Imprint Code B Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-649-18 18 in 1 CARTON Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part356 04/22/2014 Labeler - CVS Pharmacy, Inc. (062312574) Establishment Name Address ID/FEI Business Operations Bestco, Inc. 002149136 manufacture(59779-649)