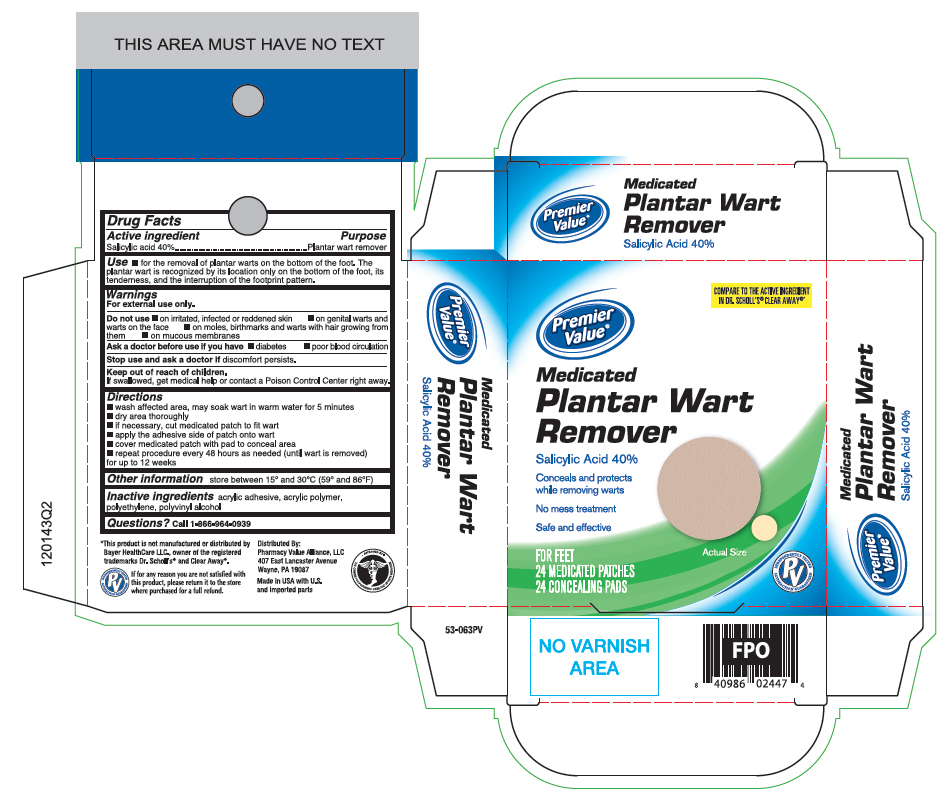
Label: SALICYLIC ACID- plantar wart remover patch
- NDC Code(s): 68016-618-00
- Packager: Chain Drug Consortium, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active ingredient
Salicylic acid 40%w/w
-
Purpose
Plantar wart remover
-
Uses
for the removal of plantar warts on the bottom of the foot. The plantar wart is recognized by its location only on the bottom of the foot, its tenderness, and the interruption of the footprint ...
-
Warnings
For external use only. Do not use - if you are diabetic or have poor blood circulation, except under the advise and supervision of a doctor or podiatrist - on irritated, infected or reddened ...
-
Directions
wash affected area, may soak wart in warm water for 5 minutes - dry area thoroughly - if necessary, cut medicated patches to fit wart - apply the adhesive side of patch onto wart - cover patch with pads ...
-
Other information
store between 59° and 86°F (15° and 30°C)
-
Inactive ingredients
acrylic adhesive, acrylic polymer, polyethylene, polyvinyl alcohol
-
Questions?
call 1-866-964-0939
-
Principal Display Panel
(What is this?)Premier Value - Medicated PLANTAR WART REMOVER - Salicylic acid 40% Conceals and protects while removing warts - No mess treatment - Safe and effective - FOR FEET - 24 MEDICATED PATCHES - 24 ...
-
INGREDIENTS AND APPEARANCEProduct Information