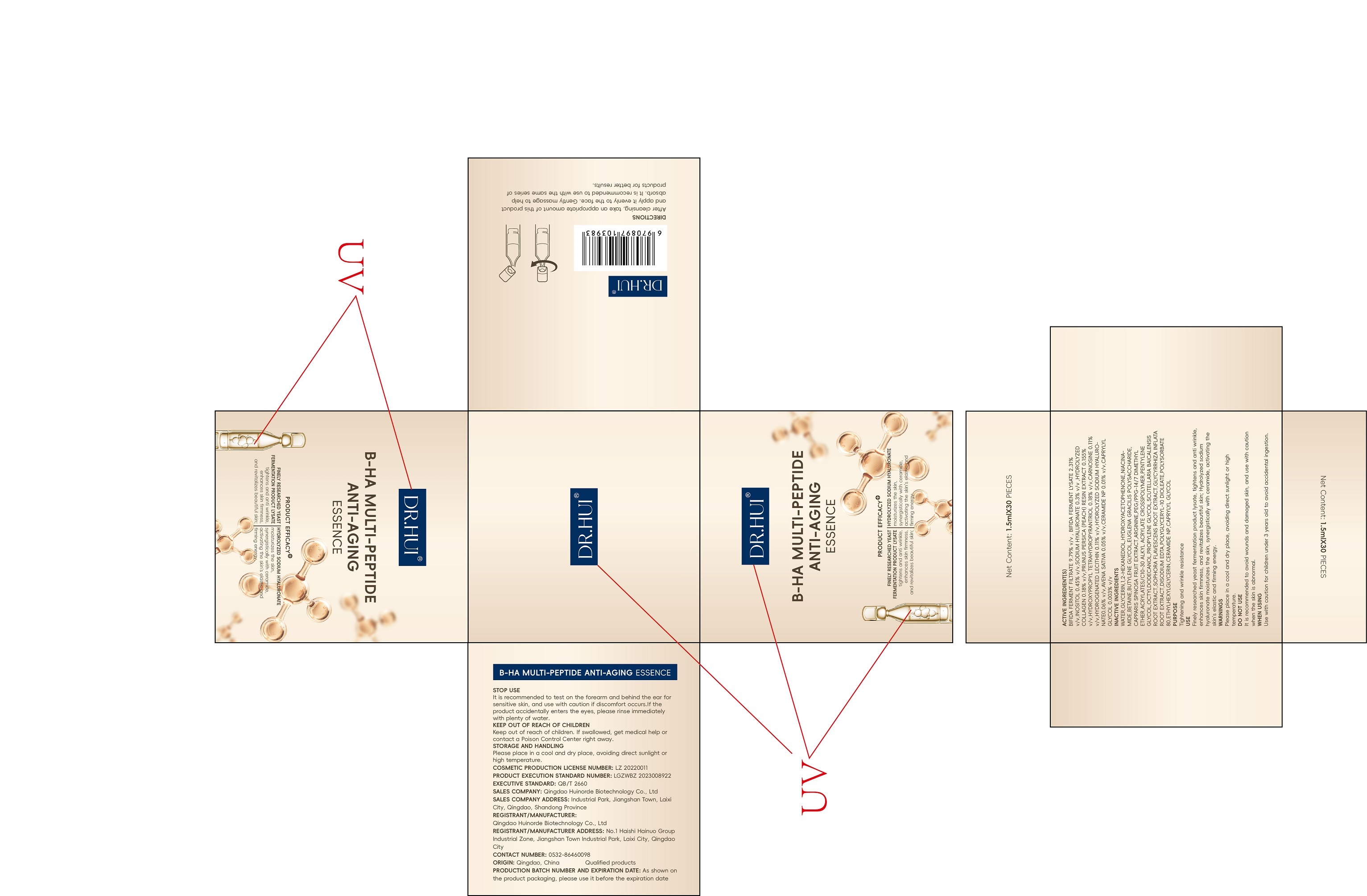
Label: DR.HUI B-HA MULTI-PEPTIDE ANTI-AGING ESSENCE- bifidobacterium bifidum solution
- NDC Code(s): 84951-001-01
- Packager: Qingdao Huinorde Biotechnology Co., LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated November 15, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
Active Ingredient
BIFIDA FERMENT FILTRATE 9.79% v/v , BIFIDA FERMENT LYSATE 2.31% v/v,INOSITOL 0.45% v/v,SODIUM HYALURONATE 0.3% v/v ,HYDROLYZED COLLAGEN 0.18% v/v,PRUNUS PERSICA (PEACH) RESIN EXTRACT 0.155% v/v,HYDROXYPROPYL TETRAHYDROPYRANTRIOL 0.18% v/v,CARNOSINE 0.11% v/v,HYDROGENATED LECITHIN 0.11% v/v,HYDROLYZED SODIUM HYALURONATE0.06% v/v,AVENA SATIVA 0.05% v/v,CERAMIDE NP 0.01% v/v,CAPRYLYL GLYCOL 0.003% v/v
- Warnings
-
INACTIVE INGREDIENTS
WATER,GLYCERIN,1,2-HEXANEDIOL,HYDROXYACETOPHENONE,NIACINAMIDE,BETAINE,BUTYLENE GLYCOL,EUGLENA GRACILIS POLYSACCHARIDE,CAPPARIS SPINOSA FRUIT EXTRACT,ARGININE,PEG/PPG-14/7 DIMETHYL ETHER,ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER,PENTYLENE GLYCOL,OCTYLDODECANOL,PROPYLENEGLYCOL,SCUTELLARIA BAICALENSIS ROOT EXTRACT,SOPHORA FLAVESCENS ROOT EXTRACT,GLYCYRRHIZA INFLATA ROOT EXTRACT,DISODIUM EDTA,POLYGLYCERYL-10 DIOLEATE,POLYSORBATE 80,ETHYLHEXYLGLYCERIN,CERAMIDE NP,CAPRYLYL GLYCOL
- Directions
- USE
- KEEP OUT OF REACH OF CHILDREN
- PURPOSE
- DO NOT USE
- WHEN USE
- STOP USE
- STORAGE AND HANDLING
- PACKAGE
-
INGREDIENTS AND APPEARANCE
DR.HUI B-HA MULTI-PEPTIDE ANTI-AGING ESSENCE
bifidobacterium bifidum solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:84951-001 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BIFIDOBACTERIUM BIFIDUM (UNII: WN5C16297P) (BIFIDOBACTERIUM BIFIDUM - UNII:WN5C16297P) BIFIDOBACTERIUM BIFIDUM 9.79 g in 100 mL BIFIDOBACTERIUM LONGUM (UNII: 831AQW699W) (BIFIDOBACTERIUM LONGUM - UNII:831AQW699W) BIFIDOBACTERIUM LONGUM 2.31 g in 100 mL INOSITOL (UNII: 4L6452S749) (INOSITOL - UNII:4L6452S749) INOSITOL 0.45 g in 100 mL HYALURONATE SODIUM (UNII: YSE9PPT4TH) (HYALURONIC ACID - UNII:S270N0TRQY) HYALURONATE SODIUM 0.3 g in 100 mL PEACH (UNII: 3OKE88I3QG) (PEACH - UNII:3OKE88I3QG) PEACH 0.155 g in 100 mL HYDROXYPROPYL TETRAHYDROPYRANTRIOL (UNII: 4U3GMG1OT1) (HYDROXYPROPYL TETRAHYDROPYRANTRIOL - UNII:4U3GMG1OT1) HYDROXYPROPYL TETRAHYDROPYRANTRIOL 0.12 g in 100 mL CARNOSINE (UNII: 8HO6PVN24W) (CARNOSINE - UNII:8HO6PVN24W) CARNOSINE 0.11 g in 100 mL HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) (HYDROGENATED SOYBEAN LECITHIN - UNII:H1109Z9J4N) HYDROGENATED SOYBEAN LECITHIN 0.11 g in 100 mL AVENA SATIVA WHOLE (UNII: 5P8D0Z74RG) (AVENA SATIVA WHOLE - UNII:5P8D0Z74RG) AVENA SATIVA WHOLE 0.05 g in 100 mL CERAMIDE NP (UNII: 4370DF050B) (CERAMIDE 3 - UNII:4370DF050B) CERAMIDE NP 0.01 g in 100 mL CAPRYLYL GLYCOL (UNII: 00YIU5438U) (CAPRYLYL GLYCOL - UNII:00YIU5438U) CAPRYLYL GLYCOL 0.003 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) NIACINAMIDE (UNII: 25X51I8RD4) BETAINE (UNII: 3SCV180C9W) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) EUGLENA GRACILIS (UNII: T426JJ26WB) CAPER BERRY (UNII: 7G4C45EE8C) ARGININE (UNII: 94ZLA3W45F) PEG/PPG-14/7 DIMETHYL ETHER (UNII: 6DNW9T7YT2) ETHER (UNII: 0F5N573A2Y) ACRYLATES/C10-30 ALKYL ACRYLATE CROSSPOLYMER (60000 MPA.S) (UNII: 8Z5ZAL5H3V) PENTYLENE GLYCOL (UNII: 50C1307PZG) OCTYLDODECANOL (UNII: 461N1O614Y) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SCUTELLARIA BAICALENSIS ROOT (UNII: 7J95K7ID2S) SOPHORA FLAVESCENS ROOT (UNII: IYR6K8KQ5K) GLYCYRRHIZA INFLATA ROOT (UNII: 1MV1Z7MKVQ) POLYGLYCERYL-10 DIOLEATE (UNII: 598RES7AXX) POLYSORBATE 80 (UNII: 6OZP39ZG8H) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:84951-001-01 30 in 1 BOX 11/15/2024 1 1.5 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 11/15/2024 Labeler - Qingdao Huinorde Biotechnology Co., LTD (541546328) Establishment Name Address ID/FEI Business Operations Qingdao Huinorde Biotechnology Co., LTD 541546328 manufacture(84951-001)