Label: DIPHENHYDRAMINE HCL solution
- NDC Code(s): 68788-8274-1
- Packager: Preferred Pharmaceuticals Inc.
- This is a repackaged label.
- Source NDC Code(s): 0904-6985
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated April 10, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each teaspoonful (5 mL))
- Purpose
- Uses
-
Warnings
Do not use
- •
- to make a child sleepy
- •
- with any other product containing diphenhydramine, even one used on skin
Ask a doctor before use if you have
- •
- a breathing problem such as emphysema or chronic bronchitis
- •
- glaucoma
- •
- difficulty in urination due to enlargement of the prostate gland
-
Directions
- •
- do not take more than 6 doses in 24 hours
- •
- mL = milliliter; FL OZ = fluid ounce
- •
- find right dose on chart below
- •
- take every 4 to 6 hours, or as directed by a doctor
Age
Dose
adults and children 12 years and over
2 - 4 teaspoonsful (25 mg to 50 mg)
children 6 to 11 years
1 - 2 teaspoonsful (12.5 mg to 25 mg)
children 2 to 5 years
do not use unless directed by a doctor
children under 2 years
do not use
- Other information
- Inactive ingredients
- Questions or comments?
-
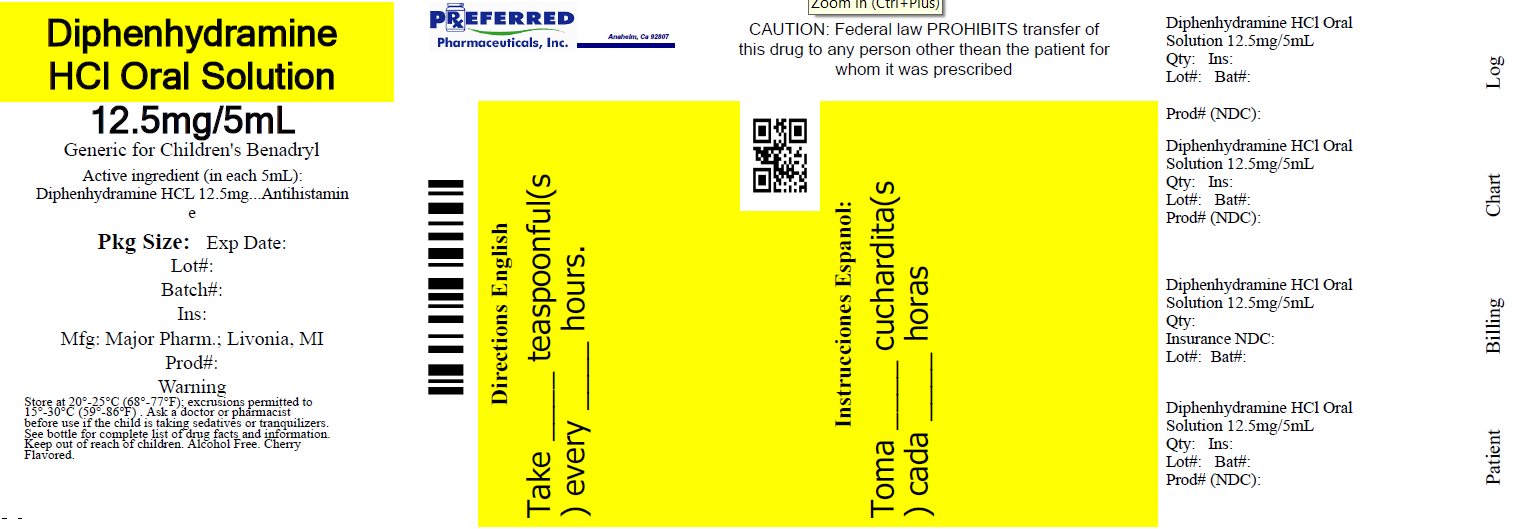
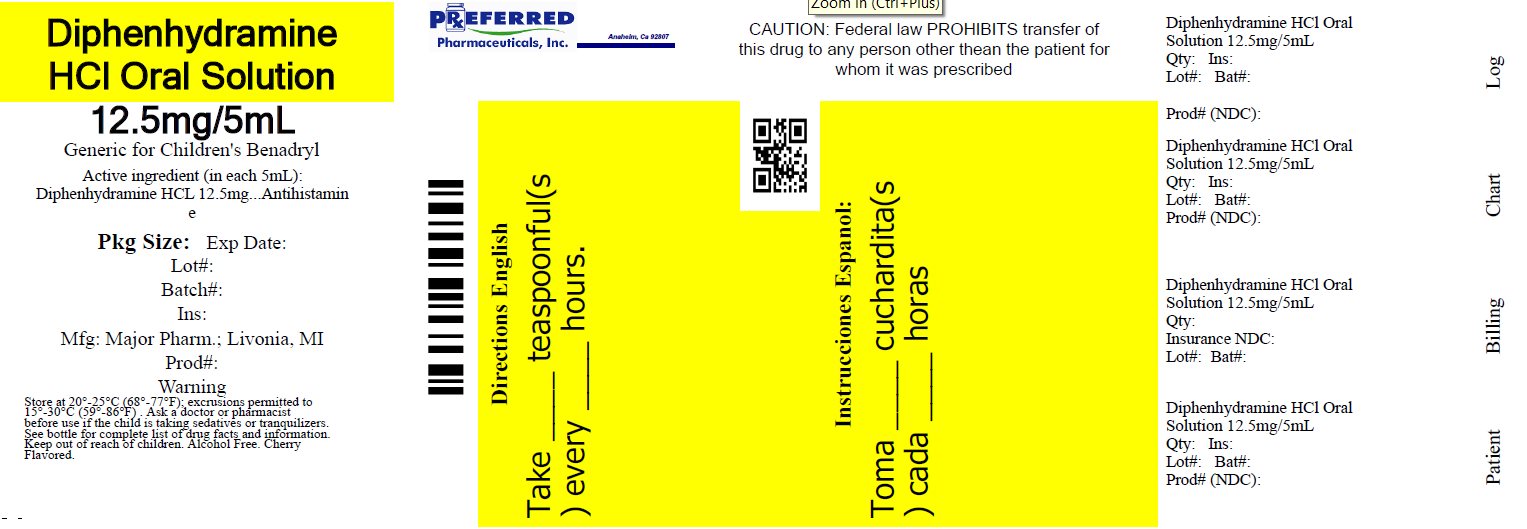
Principal display panel
MAJOR®
NDC 68788-8274-1
Relabeled By: Preferred Pharmaceuticals Inc.
Diphenhydramine HCl
Oral SolutionAntihistamine
12.5 mg/5 mL
Institutional Dispensing only
Cherry Flavored
Alcohol FreeTAMPER EVIDENT: DO NOT USE IF IMPRINTED SAFETY SEAL UNDER CAP IS BROKEN OR MISSING
50844 REV1019A01521
Distributed by: MAJOR® PHARMACEUTICALS
Livonia, MI 48152
Rev. 03/21 M-17Relabeled By: Preferred Pharmaceuticals Inc.
-
INGREDIENTS AND APPEARANCE
DIPHENHYDRAMINE HCL
diphenhydramine hcl solutionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68788-8274(NDC:0904-6985) Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 12.5 mg in 5 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) D&C RED NO. 33 (UNII: 9DBA0SBB0L) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) HIGH FRUCTOSE CORN SYRUP (UNII: XY6UN3QB6S) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SODIUM CHLORIDE (UNII: 451W47IQ8X) TRISODIUM CITRATE DIHYDRATE (UNII: B22547B95K) SUCROSE (UNII: C151H8M554) Product Characteristics Color red Score Shape Size Flavor CHERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68788-8274-1 118 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 10/14/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 341 10/14/2022 Labeler - Preferred Pharmaceuticals Inc. (791119022) Registrant - Preferred Pharmaceuticals Inc. (791119022) Establishment Name Address ID/FEI Business Operations Preferred Pharmaceuticals Inc. 791119022 RELABEL(68788-8274)