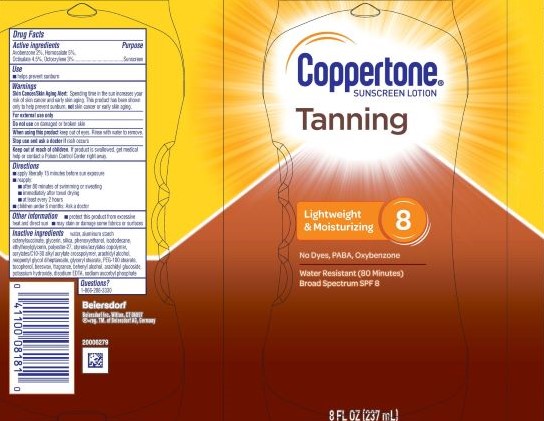
Label: COPPERTONE TANNING SUNSCREEN SPF 8- avobenzone 2%, homosalate 5%, octisalate 4.5%, octocrylene 3% lotion
- NDC Code(s): 66800-4086-1
- Packager: Beiersdorf Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated March 4, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredients
- Purpose
- Use
-
Warnings
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
For external use only
Do not use on damaged or broken skin
When using this product keep out of eyes. Rinse with water to remove.Stop use and ask a doctor if rash occurs
Keep out of reach of children. If product is swallowed, get medical help or contact a Poison Control Center right away.
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
-
Inactive ingredients
water, aluminum starch octenylsuccinate, glycerin, silica, phenoxyethanol, isododecane, ethylhexylglycerin, polyester-27, styrene/acrylates copolymer, acrylates/C10-30 alkyl acrylate crosspolymer, arachidyl alcohol, neopentyl glycol diheptanoate, glyceryl stearate, PEG-100 stearate, tocopherol, beeswax, fragrance, behenyl alcohol, arachidyl glucoside, potassium hydroxide, disodium EDTA, sodium ascorbyl phosphate
- Questions
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
COPPERTONE TANNING SUNSCREEN SPF 8
avobenzone 2%, homosalate 5%, octisalate 4.5%, octocrylene 3% lotionProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66800-4086 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 3 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.5 g in 100 g HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 5 g in 100 g Inactive Ingredients Ingredient Name Strength FRAGRANCE FLORAL ORC0902236 (UNII: R66Z4YW3X0) ISODODECANE (UNII: A8289P68Y2) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) POTASSIUM HYDROXIDE (UNII: WZH3C48M4T) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) PHENOXYETHANOL (UNII: HIE492ZZ3T) POLYESTER-7 (UNII: 0841698D2F) DOCOSANOL (UNII: 9G1OE216XY) STYRENE/ACRYLAMIDE COPOLYMER (MW 500000) (UNII: 5Z4DPO246A) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ACRYLATES CROSSPOLYMER-6 (UNII: 4GXD0Q3OS3) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) DISODIUM EDTA-COPPER (UNII: 6V475AX06U) SODIUM ASCORBYL PHOSPHATE (UNII: 836SJG51DR) PEG-100 STEARATE (UNII: YD01N1999R) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) GLYCERYL STEARATE SE (UNII: FCZ5MH785I) SYNTHETIC BEESWAX (UNII: 08MNR5YE2R) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) Product Characteristics Color white (White to Off-White) Score Shape Size Flavor Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66800-4086-1 237 g in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 11/03/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 11/03/2020 Labeler - Beiersdorf Inc (001177906)