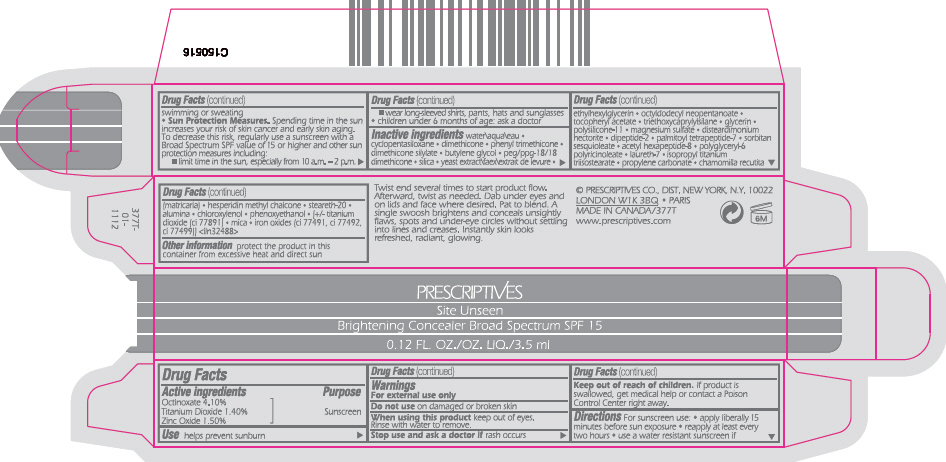
Label: PRESCRIPTIVES SITE UNSEEN BRIGHTENING CONCEALER BROAD SPECTRUM SPF 15- octinoxate, titanium dioxide, and zinc oxide liquid
-
Contains inactivated NDC Code(s)
NDC Code(s): 59158-913-01 - Packager: PRESCRIPTIVES INC.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 15, 2012
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredients
- Purpose
- Use
- Warnings
-
Directions
For sunscreen use:
- apply liberally 15 minutes before sun exposure
- reapply at least every two hours
- use a water resistant sunscreen if swimming or sweating
-
Sun Protection Measures. Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
- limit time in the sun, especially from 10 a.m. – 2 p.m.
- wear long-sleeved shirts, pants, hats and sunglasses
- children under 6 months of age: ask a doctor
-
Inactive ingredients
water\aqua\eau • cyclopentasiloxane • dimethicone • phenyl trimethicone • dimethicone silylate • butylene glycol • peg/ppg-18/18 dimethicone • silica • yeast extract\faex\extrait de levure • ethylhexylglycerin • octyldodecyl neopentanoate • tocopheryl acetate • triethoxycaprylylsilane • glycerin • polysilicone-11 • magnesium sulfate • disteardimonium hectorite • dipeptide-2 • palmitoyl tetrapeptide-7 • sorbitan sesquioleate • acetyl hexapeptide-8 • polyglyceryl-6 polyricinoleate • laureth-7 • isopropyl titanium triisostearate • propylene carbonate • chamomilla recutita (matricaria) • hesperidin methyl chalcone • steareth-20 • alumina • chloroxylenol • phenoxyethanol • [+/- titanium dioxide (ci 77891) • mica • iron oxides (ci 77491, ci 77492, ci 77499)] <iln32488>
- Other information
- SPL UNCLASSIFIED SECTION
- PRINCIPAL DISPLAY PANEL - 3.5 ml Carton
-
INGREDIENTS AND APPEARANCE
PRESCRIPTIVES SITE UNSEEN BRIGHTENING CONCEALER BROAD SPECTRUM SPF 15
octinoxate, titanium dioxide, and zinc oxide liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59158-913 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTINOXATE (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) OCTINOXATE .04551 g in 1 mL TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE .01554 g in 1 mL ZINC OXIDE (UNII: SOI2LOH54Z) (ZINC OXIDE - UNII:SOI2LOH54Z) ZINC OXIDE 0.01665 g in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) DIMETHICONE (UNII: 92RU3N3Y1O) PHENYL TRIMETHICONE (UNII: DR0K5NOJ4R) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) PEG/PPG-18/18 DIMETHICONE (UNII: 9H0AO7T794) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) YEAST (UNII: 3NY3SM6B8U) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) OCTYLDODECYL NEOPENTANOATE (UNII: X8725R883T) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) TRIETHOXYCAPRYLYLSILANE (UNII: LDC331P08E) GLYCERIN (UNII: PDC6A3C0OX) MAGNESIUM SULFATE (UNII: DE08037SAB) VALYLTRYPTOPHAN (UNII: 3G64B4AFQN) PALMITOYL TETRAPEPTIDE-7 (UNII: Q41S464P1R) SORBITAN SESQUIOLEATE (UNII: 0W8RRI5W5A) ACETYL HEXAPEPTIDE-8 (UNII: L4EL31FWIL) LAURETH-7 (UNII: Z95S6G8201) ISOPROPYL TITANIUM TRIISOSTEARATE (UNII: 949E3KBJ1I) PROPYLENE CARBONATE (UNII: 8D08K3S51E) MATRICARIA RECUTITA (UNII: G0R4UBI2ZZ) HESPERIDIN METHYLCHALCONE (UNII: 4T2GVA922X) STEARETH-20 (UNII: L0Q8IK9E08) ALUMINUM OXIDE (UNII: LMI26O6933) CHLOROXYLENOL (UNII: 0F32U78V2Q) PHENOXYETHANOL (UNII: HIE492ZZ3T) MICA (UNII: V8A1AW0880) FERRIC OXIDE RED (UNII: 1K09F3G675) FERRIC OXIDE YELLOW (UNII: EX438O2MRT) FERROSOFERRIC OXIDE (UNII: XM0M87F357) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59158-913-01 1 in 1 CARTON 1 3.5 mL in 1 TUBE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC MONOGRAPH NOT FINAL part352 11/16/2006 Labeler - PRESCRIPTIVES INC. (151701588) Establishment Name Address ID/FEI Business Operations ELGC K.K. 712808195 RELABEL(59158-913) , REPACK(59158-913) Establishment Name Address ID/FEI Business Operations Estee Lauder Pennsylvania Distribution Center 2 (PADC 2) 828534516 MANUFACTURE(59158-913) , RELABEL(59158-913) , REPACK(59158-913) Establishment Name Address ID/FEI Business Operations Estee Lauder Inc. 042918826 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS DISTRIBUTION CENTER 208579636 REPACK(59158-913) , RELABEL(59158-913) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 253616536 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD 244669714 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER COSMETICS, LTD. 205952385 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations ESTEE LAUDER N.V. 370151326 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations LEN-RON MANUFACTURING DIVISION OF ARAMIS INC 809771152 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations NORTEC KEYSTONE 943871157 MANUFACTURE(59158-913) , RELABEL(59158-913) , REPACK(59158-913) Establishment Name Address ID/FEI Business Operations NORTHTEC BRISTOL 959338336 MANUFACTURE(59158-913) , RELABEL(59158-913) , REPACK(59158-913) Establishment Name Address ID/FEI Business Operations NORTHTEC KEYSTONE 949264774 MANUFACTURE(59158-913) , RELABEL(59158-913) , REPACK(59158-913) Establishment Name Address ID/FEI Business Operations WHITMAN LABORATORIES, LTD. 216866277 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations Aveda Corporation 071352058 MANUFACTURE(59158-913) Establishment Name Address ID/FEI Business Operations Pennsylvania Logistics Center 078364654 REPACK(59158-913) , RELABEL(59158-913)