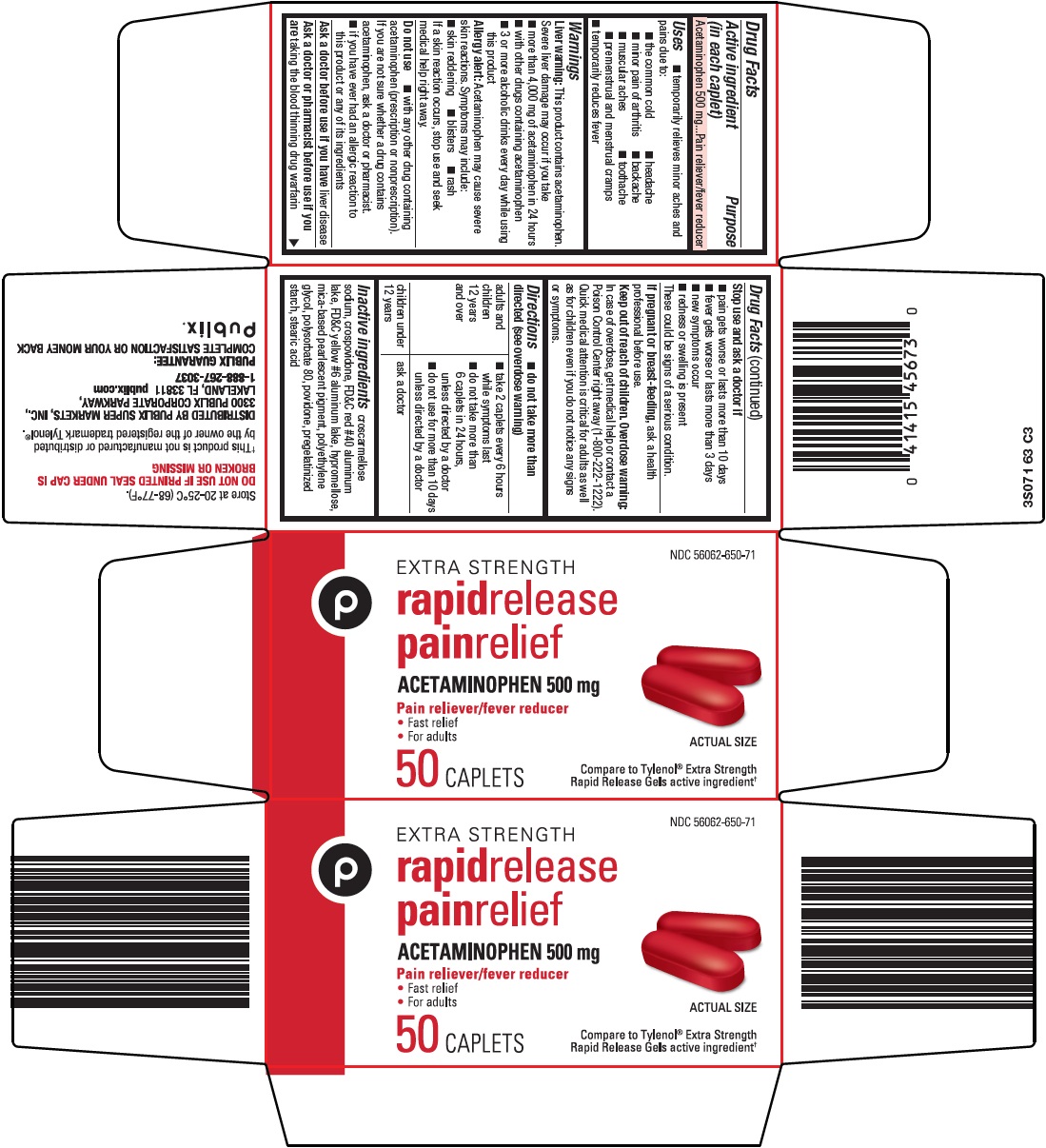
Label: RAPID RELEASE PAIN RELIEF- acetaminophen tablet, film coated
- NDC Code(s): 56062-650-71, 56062-650-78
- Packager: Publix Super Markets Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 24, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient (in each caplet)
- Purpose
- Uses
-
Warnings
Liver warning: This product contains acetaminophen. Severe liver damage may occur if you take
- •
- more than 4,000 mg of acetaminophen in 24 hours
- •
- with other drugs containing acetaminophen
- •
- 3 or more alcoholic drinks every day while using this product
Allergy alert: Acetaminophen may cause severe skin reactions. Symptoms may include:
- •
- skin reddening
- •
- blisters
- •
- rash
If a skin reaction occurs, stop use and seek medical help right away.
- Do not use
- Ask a doctor before use if you have
- Ask a doctor or pharmacist before use if you are
- Stop use and ask a doctor if
- If pregnant or breast-feeding,
- Keep out of reach of children.
-
Directions
- •
- do not take more than directed (see overdose warning)
adults and children 12 years and over
- •
- take 2 caplets every 6 hours while symptoms last
- •
- do not take more than 6 caplets in 24 hours, unless directed by a doctor
- •
- do not use for more than 10 days unless directed by a doctor
children under
12 years
ask a doctor
- Inactive ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RAPID RELEASE PAIN RELIEF
acetaminophen tablet, film coatedProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:56062-650 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ACETAMINOPHEN (UNII: 362O9ITL9D) (ACETAMINOPHEN - UNII:362O9ITL9D) ACETAMINOPHEN 500 mg Inactive Ingredients Ingredient Name Strength CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) CROSPOVIDONE (120 .MU.M) (UNII: 68401960MK) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) POLYSORBATE 80 (UNII: 6OZP39ZG8H) POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) STEARIC ACID (UNII: 4ELV7Z65AP) FD&C RED NO. 40 (UNII: WZB9127XOA) FD&C YELLOW NO. 6 (UNII: H77VEI93A8) Product Characteristics Color RED Score no score Shape OVAL Size 18mm Flavor Imprint Code 3S0 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:56062-650-71 1 in 1 CARTON 03/25/2016 1 50 in 1 BOTTLE; Type 0: Not a Combination Product 2 NDC:56062-650-78 1 in 1 CARTON 03/25/2016 2 100 in 1 BOTTLE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M013 03/25/2016 Labeler - Publix Super Markets Inc (006922009)