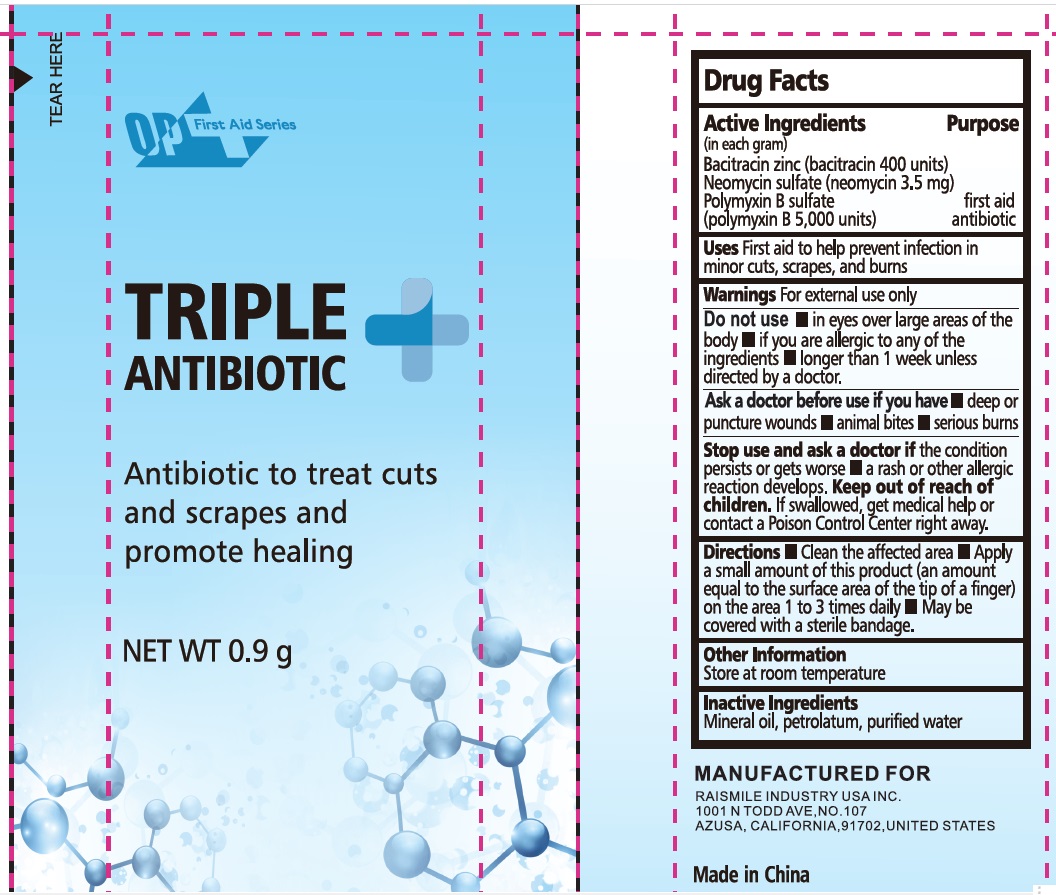
Label: OP FIRST AID SERIES TRIPLE ANTIBIOTIC- bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointment
- NDC Code(s): 43473-061-01
- Packager: Nantong Health & Beyond Hygienic Products Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 5, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredients
- Purpose
- Uses
- Warnings
- Do not use
- Ask a doctor before use if you have
- Stop use and ask a doctor if
- Keep out of reach of children
- Directions
- Other Information
- Inactive Ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
OP FIRST AID SERIES TRIPLE ANTIBIOTIC
bacitracin zinc, neomycin sulfate, polymyxin b sulfate ointmentProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:43473-061 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BACITRACIN ZINC (UNII: 89Y4M234ES) (BACITRACIN - UNII:58H6RWO52I) BACITRACIN 400 [USP'U] in 1 g NEOMYCIN SULFATE (UNII: 057Y626693) (NEOMYCIN - UNII:I16QD7X297) NEOMYCIN 0.0035 g in 1 g POLYMYXIN B SULFATE (UNII: 19371312D4) (POLYMYXIN B - UNII:J2VZ07J96K) POLYMYXIN B 5000 [USP'U] in 1 g Inactive Ingredients Ingredient Name Strength MINERAL OIL (UNII: T5L8T28FGP) PETROLATUM (UNII: 4T6H12BN9U) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:43473-061-01 0.9 g in 1 PACKET; Type 0: Not a Combination Product 12/05/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M004 12/05/2024 Labeler - Nantong Health & Beyond Hygienic Products Inc. (421280161) Registrant - Nantong Health & Beyond Hygienic Products Inc. (421280161) Establishment Name Address ID/FEI Business Operations Nantong Health & Beyond Hygienic Products Inc. 421280161 manufacture(43473-061)