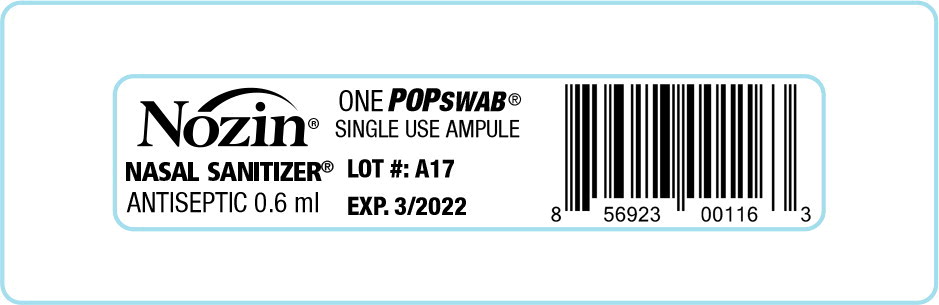
Label: NOZIN NASAL SANITIZER (ANTISEPTIC)- alcohol liquid
-
NDC Code(s):
82539-211-02,
82539-211-10,
82539-211-11,
82539-211-12, view more82539-211-62
- Packager: Global Life Technologies Corp.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 8, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Use
- Warnings
-
Directions
- wipe nostrils with tissue
- shake ampule well for 5 seconds
- remove ampule, flip and reinsert into sleeve to expose swab tip. Avoid touching swab tip.
- at dot on sleeve, squeeze firmly to crack inner ampule.
- point swab tip down, squeeze sleeve repeatedly to saturate swab tip
- Application steps: 1. Insert swab tip into RIGHT nostril. Do not go deeper than tip of swab. 2. With moderate pressure, swab around nostril eight (8) times clockwise. Cover all surfaces including inside tip of nostril. 3. squeeze to resaturate swab tip 4. insert ampule into RIGHT nostril again and swab eight (8) times counterclockwise 5. repeat steps 1, 2, 3, 4 in LEFT nostril 6. discard used ampule
- repeat all directions with second ampule, but skip tissue step.
- Other information
- Inactive ingredients
- Questions?
-
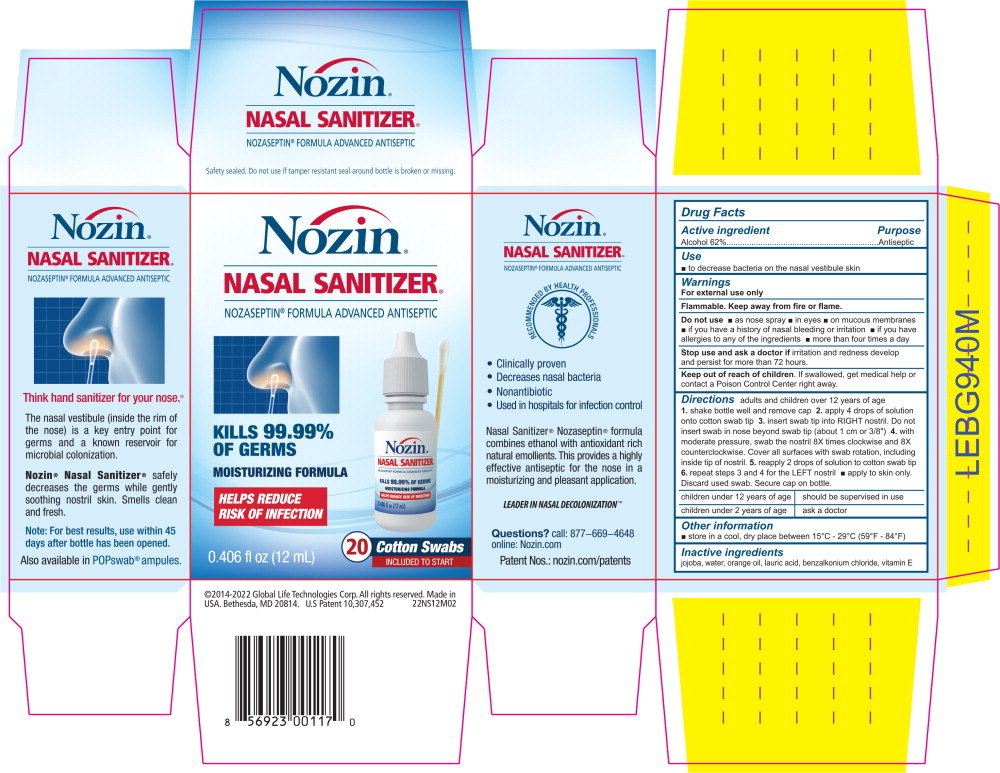
Principal Display Panel – 0.6 mL Carton Label
Nozin®
NASAL SANITIZER®
NOZASEPTIN® FORMULA ADVANCED ANTISEPTIC
KILLS 99.99% OF GERMS
MOISTURIZING FORMULA
HELPS REDUCE RISK OF INFECTION
Push in
to openPull Out Shelf
To open, push in tab above.
Pull out along perforation.RECOMMENDED BY HEALTH PROFFESSIONALS
250 single use ampules
0.020 fl oz (0.6 mL) each
5.07 fl oz (150 mL) total250 POPSWAB®
SINGLE USE AMPULES - Principal Display Panel – 0.6 mL Carton Label
-
Principal Display Panel – 0.6 mL Carton Label
Nozin®
NASAL SANITIZER®
NOZASEPTIN® FORMULA ADVANCED ANTISEPTIC
KILLS 99.99% OF GERMS
HELPS REDUCE RISK OF INFECTION
FOR PATIENTS
Ampules individually
packed and barcodedPush in
to openPull Out Shelf
To open, push in tab above.
Pull out along perforation.Popswab® ampules
packed and barcoded200 single use ampules
0.020 fl oz (0.6 mL) each
4.06 fl oz (120 mL) total - Principal Display Panel – 0.6 mL Carton Label
- Principal Display Panel – 12 mL Carton Label
-
INGREDIENTS AND APPEARANCE
NOZIN NASAL SANITIZER (ANTISEPTIC)
alcohol liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:82539-211 Route of Administration NASAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Alcohol (UNII: 3K9958V90M) (Alcohol - UNII:3K9958V90M) Alcohol 62 mL in 100 mL Inactive Ingredients Ingredient Name Strength jojoba oil (UNII: 724GKU717M) water (UNII: 059QF0KO0R) orange oil (UNII: AKN3KSD11B) lauric acid (UNII: 1160N9NU9U) benzalkonium chloride (UNII: F5UM2KM3W7) .alpha.-tocopherol acetate (UNII: 9E8X80D2L0) Product Characteristics Color Score Shape Size Flavor ORANGE (ORANGE) Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:82539-211-10 250 in 1 CASE 01/01/2017 1 1 in 1 BLISTER PACK 1 0.6 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 2 NDC:82539-211-12 2 in 1 CASE 01/01/2019 2 200 in 1 BOX 2 1 in 1 BLISTER PACK 2 0.6 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 3 NDC:82539-211-02 2 in 1 CASE 01/01/2006 3 250 in 1 BOX 3 0.6 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 4 NDC:82539-211-11 100 in 1 CASE 01/01/2019 4 2 in 1 BLISTER PACK 4 0.6 mL in 1 AMPULE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) 5 NDC:82539-211-62 12 in 1 CASE 06/01/2014 5 1 in 1 BOX 5 12 mL in 1 BOTTLE; Type 2: Prefilled Drug Delivery Device/System (syringe, patch, etc.) Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/01/2006 Labeler - Global Life Technologies Corp. (601645513)