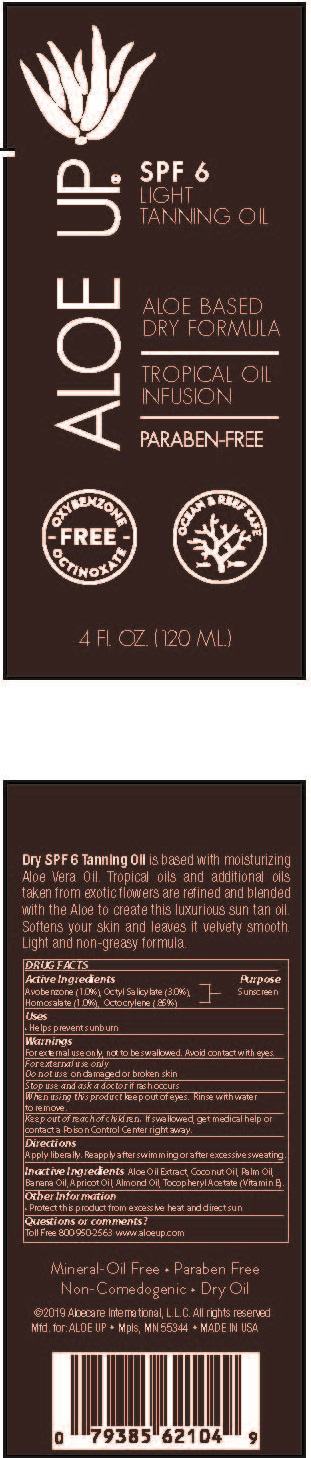
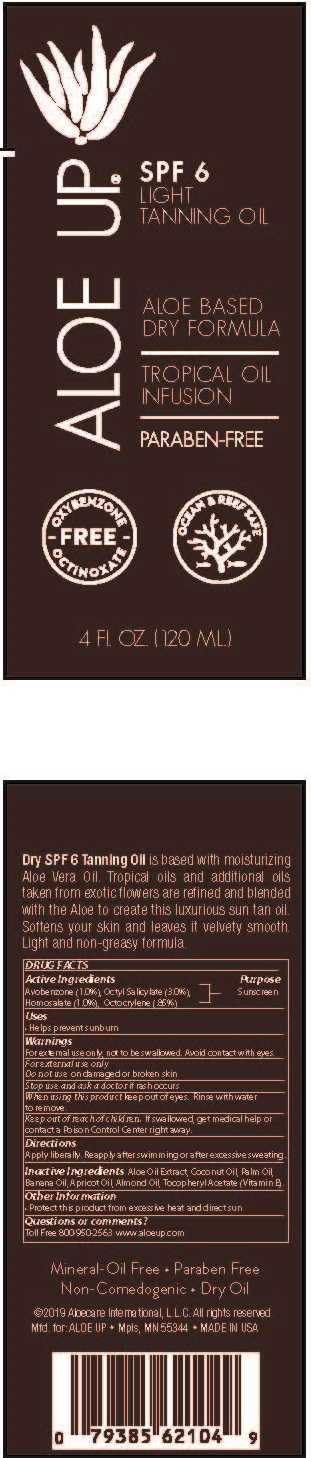
Label: ALOE UP LIGHT TANNING OIL SPF-6- avobenzone, octyl salicylate, homosalate, octocrylene spray
- NDC Code(s): 61477-226-24
- Packager: Aloe Care International, LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated February 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENTS:
- PURPOSE:
- USES
- DIRECTIONS:
- KEEP OUT OF REACH OF CHILDREN
-
WARNINGS:
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to prevent sunburn, not skin cancer or early skin aging.
For external use only.
Do not use on damaged or broken skin.
When using this product keep out of eyes. Rinse with water to remove.
Keep away from face to avoid breathing it. Contents under pressure - do not puncture or incinerate. Do not store at temperatures above 120°F.
Stop use and ask a doctor if rash occurs
Flammable: Do not use near heat, flame or while smoking. - INACTIVE INGREDIENTS
- OTHER INFORMATION
- Questions or comments?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALOE UP LIGHT TANNING OIL SPF-6
avobenzone, octyl salicylate, homosalate, octocrylene sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:61477-226 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 1 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 1 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 0.85 g in 100 mL Inactive Ingredients Ingredient Name Strength PALM OIL (UNII: 5QUO05548Z) BANANA (UNII: 4AJZ4765R9) APRICOT KERNEL OIL (UNII: 54JB35T06A) ALMOND OIL (UNII: 66YXD4DKO9) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) COCONUT OIL (UNII: Q9L0O73W7L) ALOE VERA LEAF (UNII: ZY81Z83H0X) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:61477-226-24 120 mL in 1 BOTTLE, SPRAY; Type 0: Not a Combination Product 05/17/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/17/2019 Labeler - Aloe Care International, LLC (938242187)