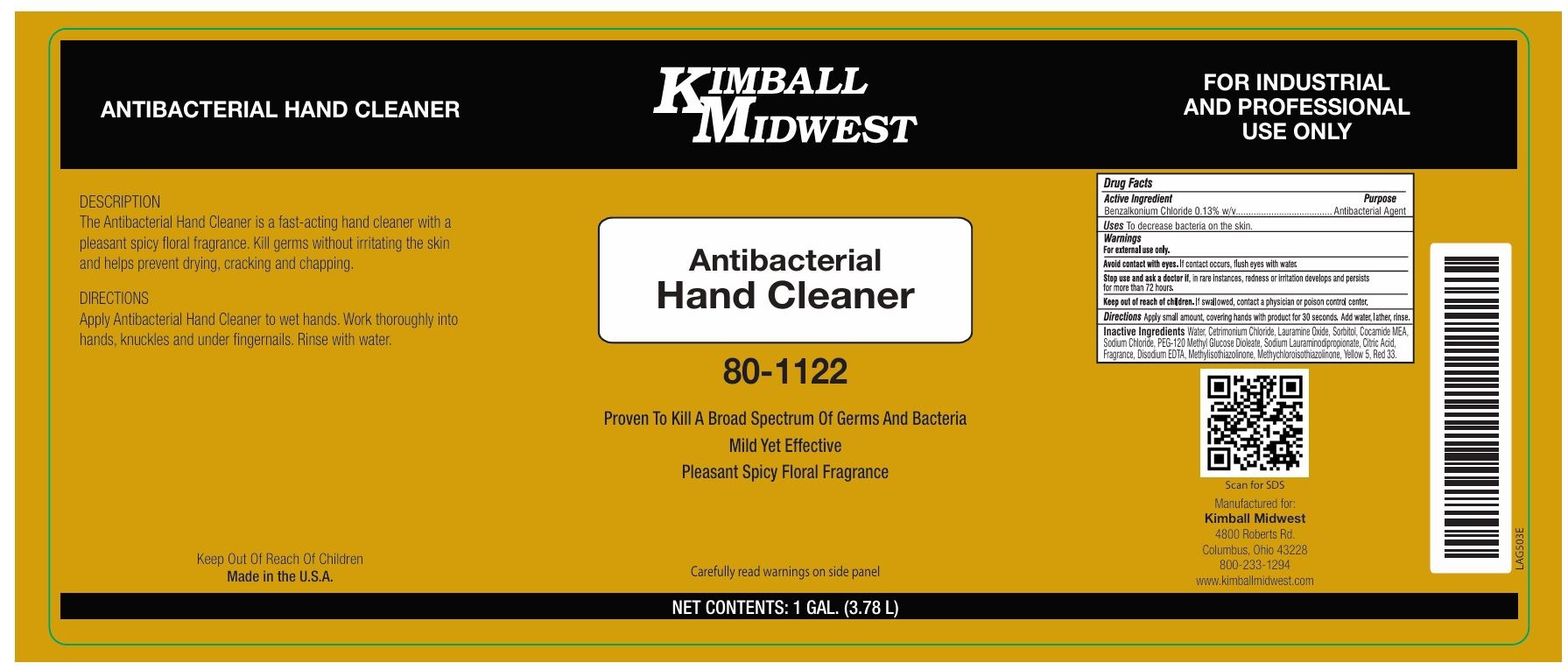
Label: KIMBALL MIDWEST ANTIBACTERIAL HAND CLEANER- benzalkonium chloride liquid
- NDC Code(s): 66608-216-01
- Packager: Kimball Midwest
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 6, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredient
- Purpose
- Uses
- Warnings
- Directions
- Inactive Ingredients
-
SPL UNCLASSIFIED SECTION
Proven To Kill A Broad Spectrum Of Germs And Bacteria
Mild Yet Effective
Pleasant Spicy Floral Fragrance
Carefully read warnings on side panel
FOR INDUSTRIAL AND PROFESSIONAL USE ONLY
Manufactured for:
Kimball Midwest
4800 Roberts Rd.
Columbus, Ohio 43228
800-233-1294
www.kimballmidwest.comDESCRIPTION
The Antibacterial Hand Cleaner is a fast-acting hand cleaner with a pleasant spicy floral fragrance. Kill germs without irritating the skin and helps prevent drying, cracking and chapping.DIRECTIONS
Apply Antibacterial Hand Cleaner to wet hands. Work thoroughly into hands, knuckles and under fingernails. Rinse with water.Made in the U.S.A.
- Packaging
-
INGREDIENTS AND APPEARANCE
KIMBALL MIDWEST ANTIBACTERIAL HAND CLEANER
benzalkonium chloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:66608-216 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 1.3 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) CETRIMONIUM CHLORIDE (UNII: UC9PE95IBP) LAURAMINE OXIDE (UNII: 4F6FC4MI8W) SORBITOL (UNII: 506T60A25R) COCO MONOETHANOLAMIDE (UNII: C80684146D) SODIUM CHLORIDE (UNII: 451W47IQ8X) PEG-120 METHYL GLUCOSE DIOLEATE (UNII: YM0K64F20V) SODIUM LAURIMINODIPROPIONATE (UNII: 7G447D0DH9) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) EDETATE DISODIUM (UNII: 7FLD91C86K) METHYLISOTHIAZOLINONE (UNII: 229D0E1QFA) METHYLCHLOROISOTHIAZOLINONE (UNII: DEL7T5QRPN) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) D&C RED NO. 33 (UNII: 9DBA0SBB0L) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:66608-216-01 3785 mL in 1 BOTTLE; Type 0: Not a Combination Product 11/13/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 11/13/2024 Labeler - Kimball Midwest (017906231)