Label: TRILOCAINE- lidocaine hydrochloride cream
- NDC Code(s): 73352-610-01
- Packager: Trifluent Pharma LLC
- Category: HUMAN PRESCRIPTION DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated June 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
-
DESCRIPTION
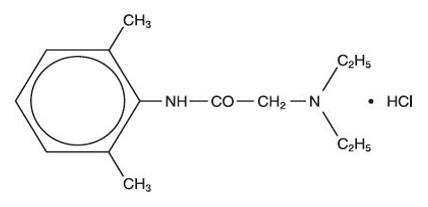
Lidocaine hydrochloride has a chemical name of 2-(diethylamino)-N-(2,6-dimethylphenyl)acetamide;hydrochloride and has the molecular weight 270.8. Lidocaine hydrocholoride (C14H22N2O ∙ HCL) has the following structural formula:
Ingredients: Each gram of Trilocaine™ 4.12% Cream contains 41.2 mg Lidocaine HCl, USP.
Inactive Ingredients include: Alkyl (C12-15) benzoate, butylparaben, Carica papaya (papaya) fruit extract, Carthamus tinctorius (safflower) seed oil, cetyl alcohol, dimethicone, disodium EDTA, emulsifying wax, ethylparaben, glycerin, glycerol stearate, hydroxypropyl cellulose, isobutylparaben, Lavandula angustifolia (lavender) oil, methylparaben, PEG 100 stearate, phenoxyethanol, propylene glycol, propylparaben, purified water, sodium lactate, xanthan gum.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
Trilocaine™ 4.12% Cream releases lidocaine to stabilize the neuronal membrane by inhibiting the ionic fluxes required for initiation and conduction of impulses, thereby effecting local anesthetic action.
Pharmacokinetics
Lidocaine may be absorbed following topical administration to mucous membranes; its rate and extent of absorption depending upon the specific site of application, duration of exposure, concentration, and total dosage. In general, the rate of absorption of local anesthetic agents following topical application occurs most rapidly after intratracheal administration. Lidocaine is also well-absorbed from the gastrointestinal tract, but little intact drug appears in the circulation because of biotransformation of the liver.
Lidocaine is metabolized rapidly by the liver, and metabolites and unchanged drug are excreted by the kidneys. Biotransformation includes oxidative N-dealkylation, ring hydroxylation, cleavage of the amide linkage, and conjugation. N-dealkylation, a major pathway of biotransformation, yields the metabolites monoethylglycinexylidide and glycinexlidide. The pharmacological/toxicological actions of these metabolites are similar to, but less potent than, those of lidocaine. Approximately 90% of lidocaine administered is excreted in the form of various metabolites and less than 10% is excreted unchanged. The primary metabolite in urine is a conjugate of 4-hydroxy-2, 6-dimethylaniline.
The plasma binding of lidocaine is dependent on drug concentration and the fraction bound decreases with increasing concentration. At concentration of 1 to 4 g of free base per mL, 60 to 80 percent of lidocaine is protein bound. Binding is also dependent on the plasma concentration of the alpha-1-acid-glycoprotein.
Lidocaine crosses the blood brain and placental barriers, presumably by passive diffusion.
Studies of lidocaine metabolism following intravenous bolus injections have shown that the elimination half-life of this agent is typically 1.5 to 2 hours. Because of the rapid rate at which lidocaine is metabolized, any condition that affects liver function may alter lidocaine kinetics. The half-life may be prolonged two-fold or more in patients with liver dysfunction. Renal dysfunction does not affect lidocaine kinetics but may increase the accumulation of metabolites.
Factors such as acidosis and the use of CNS stimulants and depressants affect the CNS levels of lidocaine required to produce overt systemic effects. Objective adverse manifestations become increasingly apparent with increasing venous plasma levels above 6 g free base per mL. In the rhesus monkey, arterial blood levels of 18-21g/mL have been shown to be threshold for convulsive activity.
- INDICATIONS
-
CONTRAINDICATIONS
Product should not be used in patients with a history of sensitivity to any of its ingredients or adverse reactions to lidocaine or amide anesthetics, which usually do not cross-react with "caine" ester type anesthetics. If excessive irritation and significant worsening occur, discontinue use and seek the advice of your physician. Product and topical lidocaine should be used cautiously in those with impaired liver function, as well as the very ill or very elderly and those with significant liver disease. Product should be used with caution in patients receiving antiarrhythmic drugs of Class I since the adverse effects are additive and generally synergistic. Product is contraindicated for tuberculous or fungal lesions of skin vaccinia, varicella and acute herpes simplex.
-
WARNINGS
For external use only. Not for ophthalmic use.
Keep out of reach of children.
Methemoglobinemia
Cases of methemoglobinemia have been reported in association with local anesthetic use. Although all patients are at risk for methemoglobinemia, patients with glucose-6-phosphate dehydrogenase deficiency, congenital or idiopathic methemoglobinemia, cardiac or pulmonary compromise, infants under 6 months of age, and concurrent exposure to oxidizing agents or their metabolites are more susceptible to developing clinical manifestations of the condition. If local anesthetics must be used in these patients, close monitoring for symptoms and signs of methemoglobinemia is recommended.
Signs and symptoms of methemoglobinemia may occur immediately or may be delayed some hours after exposure and are characterized by a cyanotic skin discoloration and abnormal coloration of the blood. Methemoglobin levels may continue to rise; therefore, immediate treatment is required to avert more serious central nervous system and cardiovascular adverse effects, including seizures, coma, arrhythmias, and death.
Discontinue [the use of this product] and any other oxidizing agents. Depending on the severity of the symptoms, patients may respond to supportive care, i.e., oxygen therapy, hydration. More severe symptoms may require treatment with methylene blue, exchange transfusion, or hyperbaric oxygen.
DRUG INTERACTIONS
Patients that are administered local anesthetics may be at increased risk of developing methemoglobinemia when concurrently exposed to the following oxidizing agents:
Class Examples Nitrates/Nitrites nitric oxide, nitroglycerin, nitroprusside, nitrous oxide Local anesthetics Articaine, benzocaine, bupivacaine, lidocaine, mepivacaine, prilocaine, procaine, ropivacaine, tetracaine Antineoplastic agents cyclophosphamide, flutamide, hydroxyurea, ifosfamide, rasburicase Antibiotics dapsone, nitrofurantoin, para-aminosalicylic acid sulfonamides Antimalarials chloroquine, primaquine Anticonvulsants Phenobarbital, phenytoin, sodium valproate Other drugs acetaminophen, metoclopramide, quinine, sulfa drugs (i.e., sulfasalazine) PATIENT COUNSELING INFORMATION
Inform patients that use of local anesthetics may cause methemoglobinemia, a serious condition that must be treated promptly. Advise patients or caregivers to stop use and seek immediate medical attention if they or someone in their care experience the following signs or symptoms: pale, gray, or blue colored skin (cyanosis); headache; rapid heart rate; shortness of breath; lightheadedness; or fatigue.
Topical formulations of lidocaine may be absorbed to a greater extent through mucous membranes and abraded, fissured or irritated skin than through intact skin. Product should not be ingested or applied into the mouth, inside of the nose or in the eyes. Product should not be used in the ears. Any situation where lidocaine penetrates beyond the tympanic membrane into the middle ear is contraindicated because of ototoxicity associated with lidocaine observed in animals when instilled in the middle ear. Product should not come into contact with the eye or be applied into the eye because of the risk of severe eye irritation and the loss of eye surface sensation, which reduces protective reflexes and can lead to corneal irritation and possibly abrasion. If eye contact occurs, rinse out the eye immediately with saline or water and protect the eye surface until sensation is restored.
-
PRECAUTIONS
If irritation or sensitivity occurs or infection appears, discontinue use and institute appropriate therapy. If extensive areas are treated, the possibility of systemic absorption exists. Trilocaine™ 4.12% Cream should be used with caution in ill, elderly, debilitated patients and children who may be more sensitive to the systemic effects of lidocaine.
CARCINOGENESIS, MUTAGENESIS AND IMPAIRMENT OF FERTILITY
Studies of lidocaine in animals to evaluate the carcinogenic and mutagenic potential of the effect on fertility have not been conducted.
USE IN PREGNANCY
Teratogenic Effects
Pregnancy Category B
Reproduction studies have been performed for lidocaine in rats at doses up to 6.6 times the human dose and have revealed no evidence of harm to the fetus caused by lidocaine. There are, however, no adequate and well-controlled studies in pregnant women. Animal reproduction studies are not always predictive of human response. General consideration should be given to this fact before administering lidocaine to women of childbearing potential, especially during early pregnancy when maximum organogenesis takes place.
-
ADVERSE REACTIONS
During or immediately after treatment, the skin at the site of treatment may develop erythema or edema or may be the locus of abnormal sensation.
To report SUSPECTED ADVERSE REACTIONS, contact Trifluent Pharma at (210) 944-6920 or the FDA at 1-800-FDA-1088 or www.fda.gov/medwatch for voluntary reporting of adverse reactions.
- DOSAGE AND ADMINISTRATION
- HOW SUPPLIED
- SPL UNCLASSIFIED SECTION
-
PRINCIPAL DISPLAY PANEL - 28.3 g Tube Pouch Label
NDC: 73352-610-01
Rx only
TRIFLUENT
PHARMA™Trilocaine™
(4.12% Lidocaine HCl Cream)
Topical Anesthetic
Net Wt. 1 oz. (28.3 g)Active ingredient: Lidocaine HCl, USP 4.12%
Inactive ingredients: Alkyl (C12-15) benzoate, butylparaben, Carica papaya (papaya) fruit extract,
Carthamus tinctorius (safflower) seed oil, cetyl alcohol, dimethicone, disodium EDTA, emulsifying wax,
ethylparaben, glycerin, glycerol stearate, hydroxypropyl cellulose, isobutylparaben, Lavandula
angustifolia (lavender) oil, methylparaben, PEG 100 stearate, phenoxyethanol, propylene glycol,
propylparaben, purified water, sodium lactate, xanthan gum.Indications: For the temporary relief of pain and itching associated with minor burns, sunburn, minor
cuts, scrapes, insect bites, and minor skin irritation.KEEP THIS AND ALL MEDICATIONS OUT OF REACH OF CHILDREN
Usual Adult Dosage: Apply a thin film to the affected area two or three times daily or as directed by a
physician.Additional Product Information Enclosed.
Caution: For external use only. Not for ophthalmic use.
Store at 20°-25°C (68°-77°F)
[see USP Controlled Room Temperature]. Protect from freezing.Distributed By:
Trifulent Pharma
San Antonio, TX 78213610-01
Rev. 03/2024FPO
Data Matrix
-
INGREDIENTS AND APPEARANCE
TRILOCAINE
lidocaine hydrochloride creamProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:73352-610 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE HYDROCHLORIDE (UNII: V13007Z41A) (LIDOCAINE - UNII:98PI200987) LIDOCAINE HYDROCHLORIDE ANHYDROUS 41.2 mg in 1 g Inactive Ingredients Ingredient Name Strength ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) BUTYLPARABEN (UNII: 3QPI1U3FV8) PAPAYA (UNII: KU94FIY6JB) SAFFLOWER OIL (UNII: 65UEH262IS) CETYL ALCOHOL (UNII: 936JST6JCN) DIMETHICONE (UNII: 92RU3N3Y1O) EDETATE DISODIUM (UNII: 7FLD91C86K) ETHYLPARABEN (UNII: 14255EXE39) GLYCERIN (UNII: PDC6A3C0OX) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) ISOBUTYLPARABEN (UNII: 0QQJ25X58G) LAVENDER OIL (UNII: ZBP1YXW0H8) METHYLPARABEN (UNII: A2I8C7HI9T) PHENOXYETHANOL (UNII: HIE492ZZ3T) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) WATER (UNII: 059QF0KO0R) SODIUM LACTATE (UNII: TU7HW0W0QT) XANTHAN GUM (UNII: TTV12P4NEE) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73352-610-01 1 in 1 POUCH 06/24/2024 1 28.3 g in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date UNAPPROVED DRUG OTHER 06/24/2024 Labeler - Trifluent Pharma LLC (117167281)

