Label: X-PUR OPTI-RINSE PLUS- anticavity fluoride rinse mouthwash
-
Contains inactivated NDC Code(s)
NDC Code(s): 77982-114-11 - Packager: Laboratoires MSP Inc
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated October 29, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Adults and children 6 years and older:
Use once a day after brushing your teeth with a toothpaste
Remove cap
Pour 10 milliliters into a cap (10 mL mark on the inside of cap); do not fill above 10 mL mark Vigorously swish 10 milliliters of rinse between your teeth for 1 minute and then spit outDo not swallow the rinse
Do not eat or drink for 30 minutes after rinsing
Instruct children under 12 years of age in good brushing and rinsing habits (to minimize swallowing)
Supervise children as necessary until capable of using without supervision- Children 6 years and under
Consult a dentist or doctor
- Inactive ingredients
- OTHER SAFETY INFORMATION
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
X-PUR OPTI-RINSE PLUS
anticavity fluoride rinse mouthwashProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:77982-114 Route of Administration DENTAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SODIUM FLUORIDE (UNII: 8ZYQ1474W7) (FLUORIDE ION - UNII:Q80VPU408O) SODIUM FLUORIDE 0.05 g in 100 mL Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) BENZOIC ACID (UNII: 8SKN0B0MIM) XYLITOL (UNII: VCQ006KQ1E) POLOXAMER 407 (UNII: TUF2IVW3M2) CETYLPYRIDINIUM CHLORIDE (UNII: D9OM4SK49P) MINT (UNII: FV98Z8GITP) GLYCERIN (UNII: PDC6A3C0OX) CITRUS BIOFLAVONOIDS (UNII: BD70459I50) WATER (UNII: 059QF0KO0R) SODIUM PHOSPHATE (UNII: SE337SVY37) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:77982-114-11 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 06/18/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part355 06/18/2021 Labeler - Laboratoires MSP Inc (251046124)

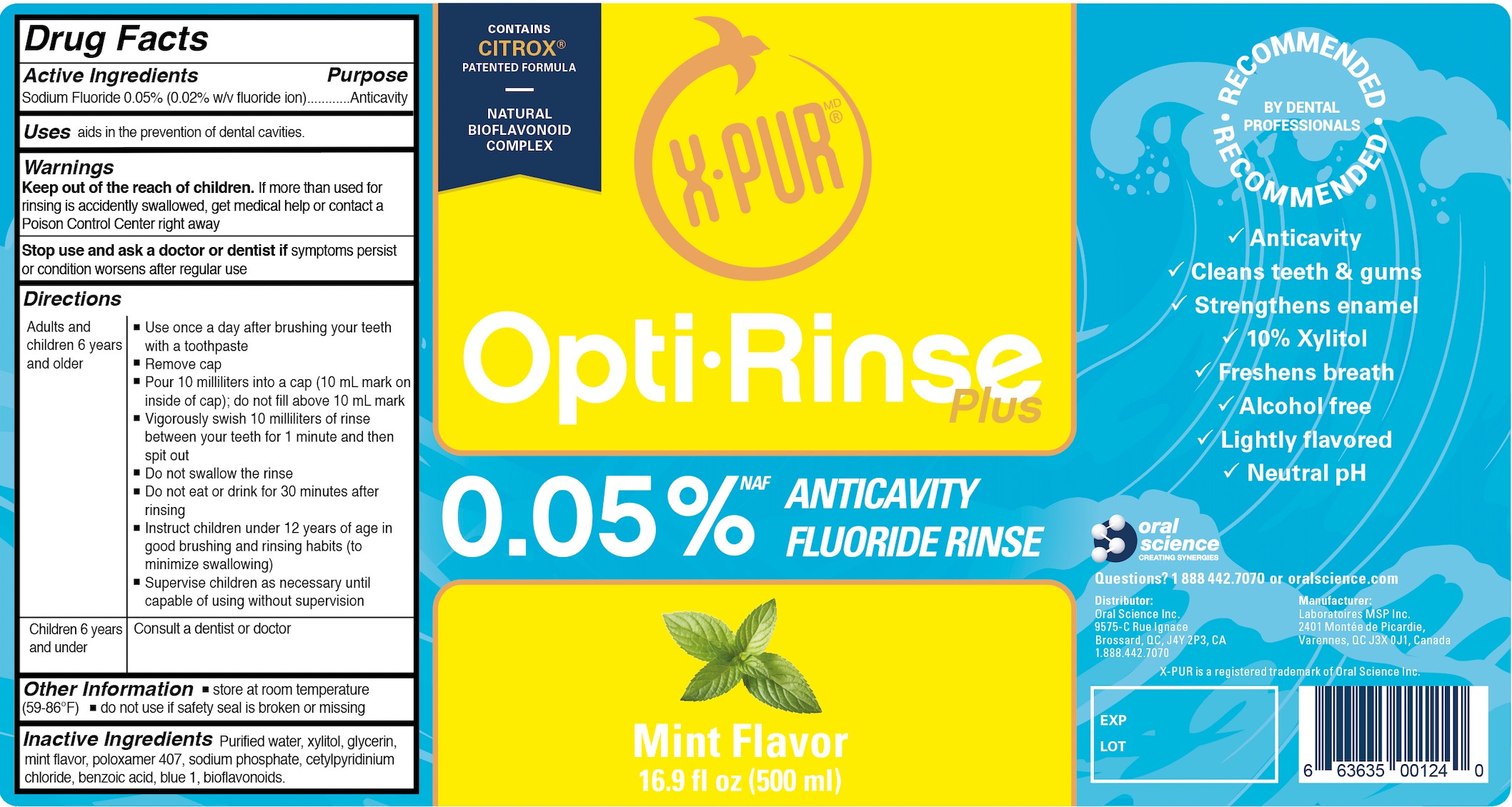
 500 mL NDC: 77982-114-11
500 mL NDC: 77982-114-11