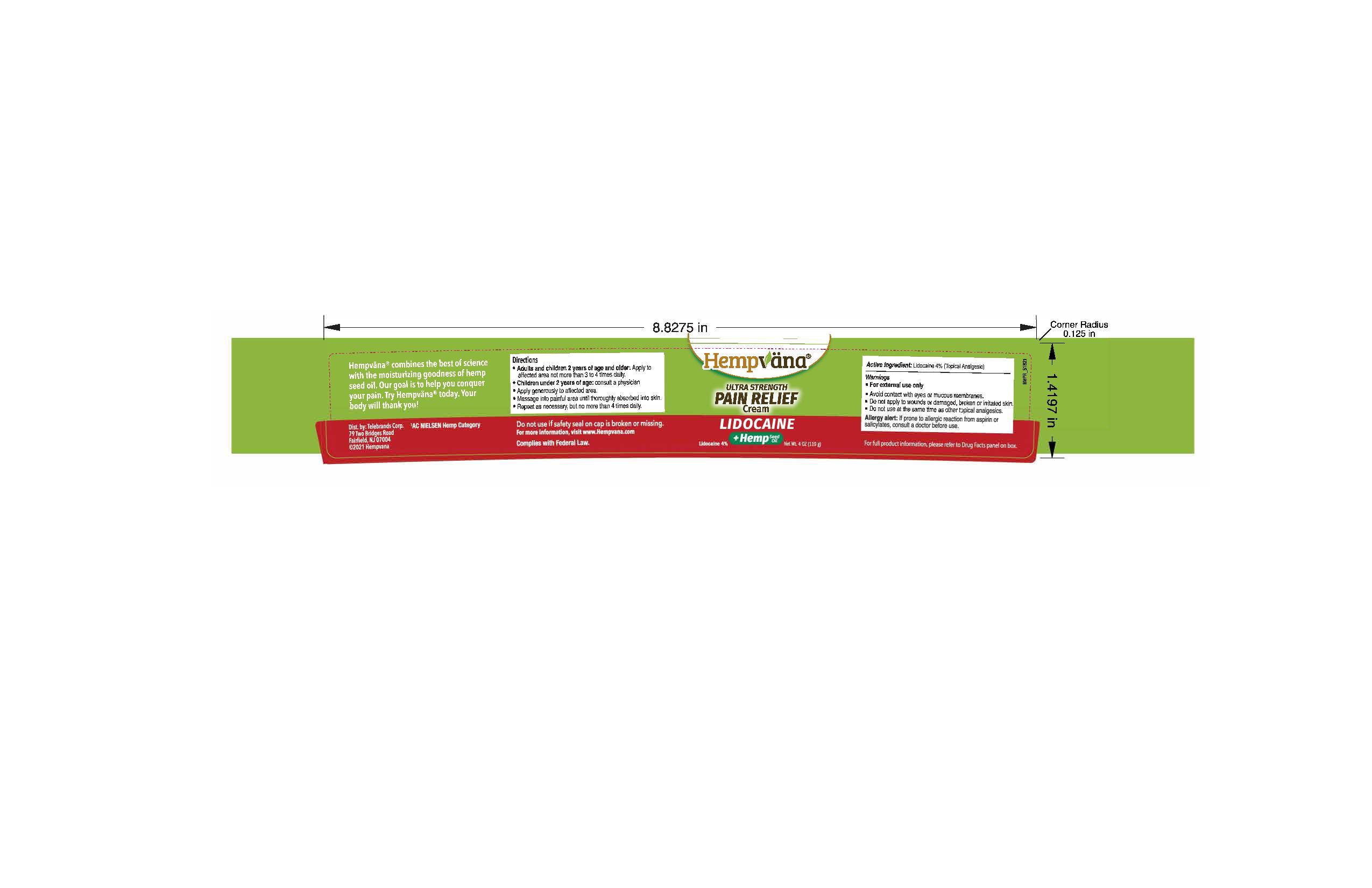
Label: HEMPVANA ULTRA STRENGTH PAIN RELIEF CREAM- lidocaine 4% cream
- NDC Code(s): 73287-016-01
- Packager: Telebrands Corp
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated May 24, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- Purpose
- Uses
-
Warnings
For external use only
Allergy alert: If prone to allergic reaction from aspirin or salicylates, consult a doctor before use.
When using this product
- Use only as directed. Read and follow all directions and warnings on this label.
- Do not bandage tightly or apply local heat (such as heating pads) to the area of use.
- Avoid contact with eyes or mucous membranes.
- Do not apply to wounds or damaged, broken or irritated skin.
- Do not use at the same time as other topical analgesics.
-
Directions
- Adults and children 2 years of age and older: Apply to affected area not more than 3 to 4 times daily.
- Children under 2 years of age: consult a physician
- Apply generously to affected area.
- Massage into painful area until thoroughly absorbed into skin.
- Repeat as necessary, but no more than 4 times daily.
-
Inactive Ingredients
Water/Aqua/Eau, Ethylhexyl Stearate, Butylene Glycol, Dimethicone, Stearic Acid, Caprylic/Capric Triglyceride, Cannabis Sativa (Hemp) Seed Oil, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, Helianthus Annuus (Sunflower) Seed Oil, Curcuma Longa (Turmeric) Root Extract, Allantoin, Glycerin, Tocopheryl Acetate, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Phenoxyethanol, Caprylyl Glycol, Ethylhexylglycerin, Hexylene Glycol, Sodium Hydroxide, Disodium EDTA, Sodium Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Polysorbate 80, Blue 1 (CI 42090), Yellow 5 (CI 19140)
- Questions?
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
HEMPVANA ULTRA STRENGTH PAIN RELIEF CREAM
lidocaine 4% creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:73287-016 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength LIDOCAINE (UNII: 98PI200987) (LIDOCAINE - UNII:98PI200987) LIDOCAINE 4 g in 100 g Inactive Ingredients Ingredient Name Strength FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C YELLOW NO. 5 (UNII: I753WB2F1M) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) ALLANTOIN (UNII: 344S277G0Z) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) CARBOMER INTERPOLYMER TYPE A (ALLYL SUCROSE CROSSLINKED) (UNII: 59TL3WG5CO) PHENOXYETHANOL (UNII: HIE492ZZ3T) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) ISOHEXADECANE (UNII: 918X1OUF1E) POLYSORBATE 80 (UNII: 6OZP39ZG8H) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) WATER (UNII: 059QF0KO0R) ETHYLHEXYL STEARATE (UNII: EG3PA2K3K5) DIMETHICONE (UNII: 92RU3N3Y1O) STEARIC ACID (UNII: 4ELV7Z65AP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) CANNABIS SATIVA SEED OIL (UNII: 69VJ1LPN1S) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) SUNFLOWER OIL (UNII: 3W1JG795YI) TURMERIC (UNII: 856YO1Z64F) GLYCERIN (UNII: PDC6A3C0OX) CAPRYLYL GLYCOL (UNII: 00YIU5438U) HEXYLENE GLYCOL (UNII: KEH0A3F75J) SODIUM HYDROXIDE (UNII: 55X04QC32I) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) SODIUM ACRYLATE/SODIUM ACRYLOYLDIMETHYLTAURATE COPOLYMER (4000000 MW) (UNII: 1DXE3F3OZX) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:73287-016-01 1 in 1 CARTON 05/24/2021 1 119 g in 1 JAR; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 05/24/2021 Labeler - Telebrands Corp (177266558) Establishment Name Address ID/FEI Business Operations Neutraderm, Inc. 146224444 manufacture(73287-016)