Label: GRAY MALIN KIT- avobenzone, homosalate, octisalate, octocrylene kit
-
Contains inactivated NDC Code(s)
NDC Code(s): 75936-244-01, 75936-245-01, 75936-246-01, 75936-247-01, view more75936-248-01, 75936-249-01, 75936-253-01 - Packager: TAYLOR JAMES, LTD.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated November 16, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Play Everyday Sunscreen SPF 50 with Sunflower extract Active ingredients
- Defense Refresh (Re)setting Mist SPF 40
- Unseen Sunscreen SPF 40 active ingredients
- PURPOSE
- KEEP OUT OF REACH OF CHILDREN
- INDICATIONS & USAGE
- WARNINGS
-
Play Everyday Lotion SPF 50 with Sunflower Extract Directions
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Reapply:after 80 minutes of swimming or sweating
immediately after towel drying
at least every 2 hours- Sun Protection Measures. Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with broad spectrum SPF of 15 or higher and other sun protection measures
including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear Long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
DOSAGE & ADMINISTRATION
Directions
- Apply generously and evenly 15 minutes before sun exposure
- Reapply:after 40 minutes of swimming or sweating
immediately after towel drying
at least every 2 hours- Sun Protection Measures. Spending time in the sun increases your risk of skin
cancer and early skin aging. To decrease this risk, regularly use a sunscreen
with broad spectrum SPF of 15 or higher and other sun protection measures
including:- limit time in the sun, especially from 10 a.m. - 2 p.m.
- Wear Long-sleeved shirts, pants, hats, and sunglasses
- Children under 6 months: Ask a doctor
-
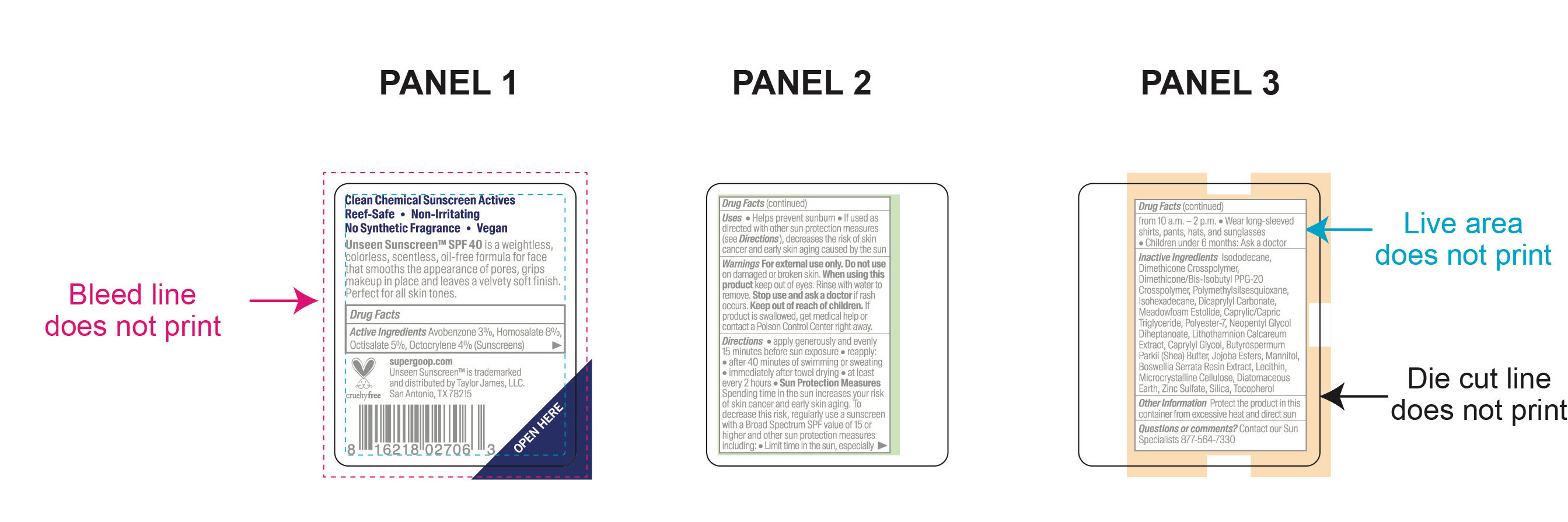
Unseen Sunscreen SPF 40 Inactive Ingredients
Inactive Ingredients
Isododecane, Dimethicone Crosspolymer, Dimethicone/Bis-Isobutyl PPG-20 Crosspolymer, Polymethylsilsesquioxane, Isohexadecane, Dicrapylyl Carbonate, Meadowfoam Estolide, Caprylic/Capric Triglyceride, Polyester-7, Neopentyl Glycol Diheptanoate, Lithothamnion Calcareum Extract,Butyrospermum Parkii (Shea) Butter, Jojoba Esters, Mannitol, Boswellia Serrata Resin Extract, Lecithin, Microcrystalline Cellulose, Diatomaceous Earth, Zinc Sulfate, Silica, Tocopherol
-
Defense Refresh (Re)setting Mist SPF 40 Inactive Ingredients
Inactive Ingredients
Alcohol Denat., Bisabolol, Brassica Campestris/Aleurites Fordi Oil Copolymer, Butyloctyl Salicylate, Caprylic/Capric Triglyceride, Capryloyl Glycerin/Sebacic Acid Copolymer, Dicaprylyl Carbonate, Diethylhexyl Syringylidenemalonate, Diheptyl Succinate, Diisooctyl Succinate, Ethyl Ferulate, Isododecane, Lauroyl Lysine, Mentha Piperita (Peppermint) Oil, Mentha Viridis (Spearmint) Leaf Oil, Nylon-12, PVP, Rosmarinus Officinalis (Rosemary) Leaf Oil, Silica Silylate
-
Play Everyday Sunscreen SPF 50 with sunflower Inactive ingredients
Inactive ingredients
Water, Acrylates Copolymer, Diisopropyl Sebacate, Glycerin, Isodecyl Neopentanoate, Isododecane, Lauryl Lactate, Cetyl Alcohol, Potassium Cetyl Phosphate, Brassica Camprestris/Aleurites Fordi Copolymer, Oryza Sativa (Rice) Bran Extract, Cetearyl Olivate, Ammonium Acryloyldimethyltaurate/VP Copolymer, Hydroxyacetophenone, Sorbitan Olivate, Diethylhexyl Syringylidenemalonate, Aniba Rosaeodora (Rosewood) Wood Oil, Chlorphenesin, Citrus Aurantium Dulcis (Orange) Peel Oil, Citrus Limon (Lemon) Peel Oil, Ethylhexylglycerin, Eucalyptus Globulus Leaf Oil, Ocimum Bascilicum (Basil) Flower/Leaf Extract, Pelargonium Graveolens Flower Oil, Pogostemon Cablin Oil, Pentylene Glycol, 1,2-hexanediol, Caprylyl Glycol, Xanthan Gum, Helianthus Annuus (Sunflower) Extract, Behenic Acid, Cetyl Behenate, Isostearyl Isostearate, Trisodium Ethylenediamene Disuccinate, Tocopherol, Allantoin, Rosmarinus Officinalis (Rosemary) Leaf Extract, Caprylic/Capric Triglyceride,Panthenol, Pentasodium Triphosphate, Citric Acid
-
Antioxidant body mist SPF 50 Vitamin C
Inactive Ingredients
Alcohol Denat., Butyloctyl Salicylate, Camellia Sinensis Leaf Extract, Cananga Odorata Flower Oil, Caprylic/Capric Triglyceride, Citrus Grandis (Grapfruit) Peel Oil, Citrus medica Limonium (Lemon) Oil, Citrus Nobilis (Mandarin Orange) Peel Oil, Citrus reticulata (Tangerine) Leaf Oil, Citrus Sinensis peel Oil, Dicaprylyl Carbonate, Diisopropyl adiipate, Ethyl Ferulate, Euterpe Oleracea Fruit Extract, Helianthuus Annuus (Sunflower) Seed Oil, Isodecyl Neopentanoate, Lavandula Angustifolia (Lavender) Oil, Mentha Piperita (Peppermint) Oil, Mentha Viridis(Spearmint) Tetrahexyldecyl Ascorbate, Tridecyl Neopentanoate, VA/Butyl Maleate/ Isobornyl Acrylate Copolymer, Vitis Vinifera (Grape) Seed Oil
-
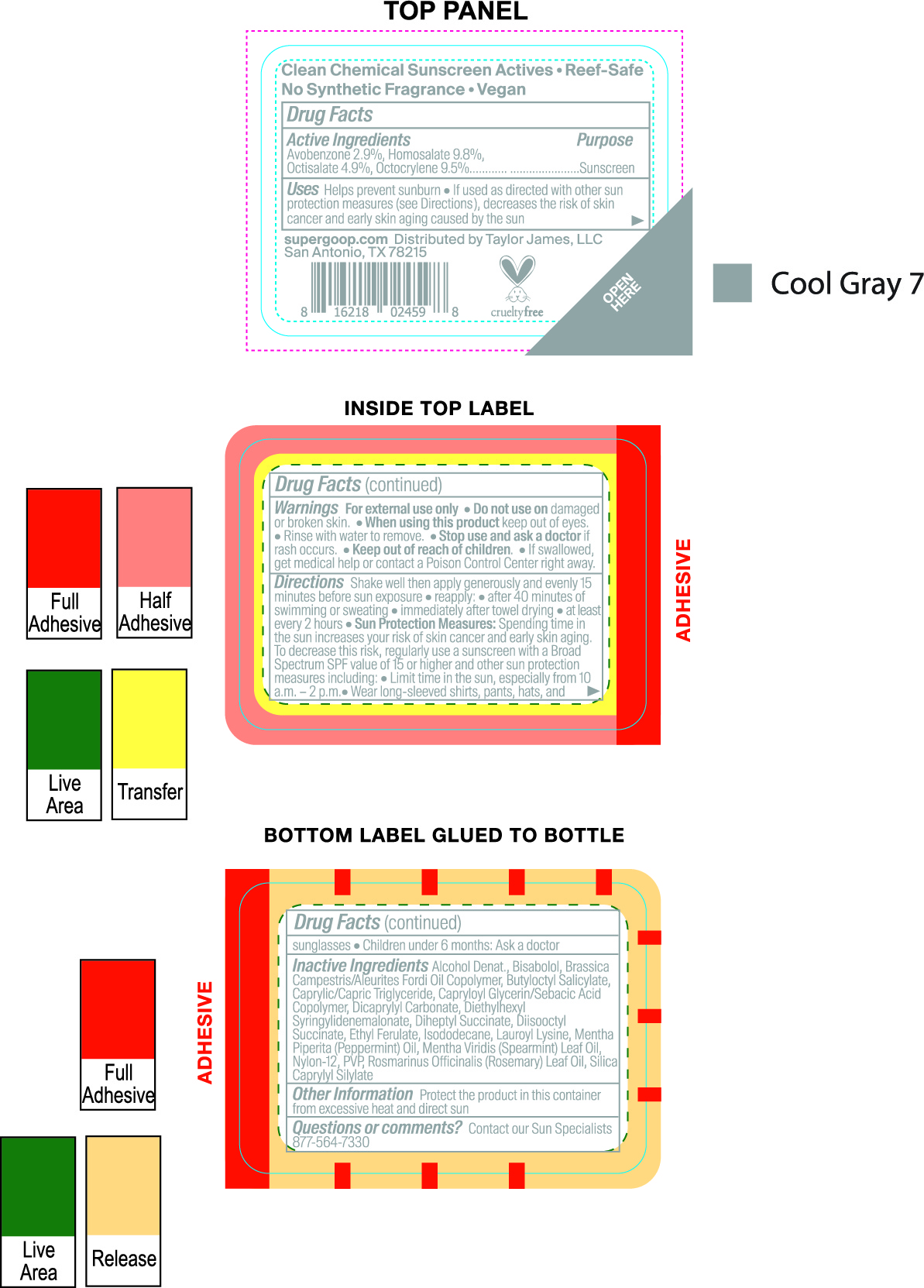
Glow Oil SPF 50
Inactive Ingredients
Caprylic/Capric Triglyceride, Alcohol, Octyldodecanol, C12-15 Alkyl Benzoate, Polyester-8, Polyamide-3, Lauryl Lactate, Isodecyl Neopentanoate, Diisopropyl Sebacate, Diethylhexylsyringylidenemalonate, Tocopherol, Glycine Soja (Soybean)Oil, Vitis Vinifera (grape) Seed Oil, Raphanus Sativus (Radish) Seed Oil, Limnanthes Alba(Meadowfoam) Seed Oil, Helianthus Annuus (Sunflower) Seed Oil, Cocos Nucifera (coconut) Oil, Butyrospermum Parkii (Shea) Butter, Argania Spinosa Kernel Oil, Pentaerythrityl Tetra-di-t-butyl hydroxyhydrocinnamate. Alaria Esculanta Extract, t-Butyl Alcohol, Cucumis Sativus(cucumber) Fruit Extract, Denatonium Benzoate, Citric Acid
- Lip Shield SPF 30 with mint
- Antioxidant Body Mist With vitamin C SPF 30
- Glow Oil SPF 50
- Unseen Sunscreen SPF 40
- Defense Refresh (Re) Setting Mist SPF 40
- Everyday Lotion with Sunflower Extract
-
INGREDIENTS AND APPEARANCE
GRAY MALIN KIT
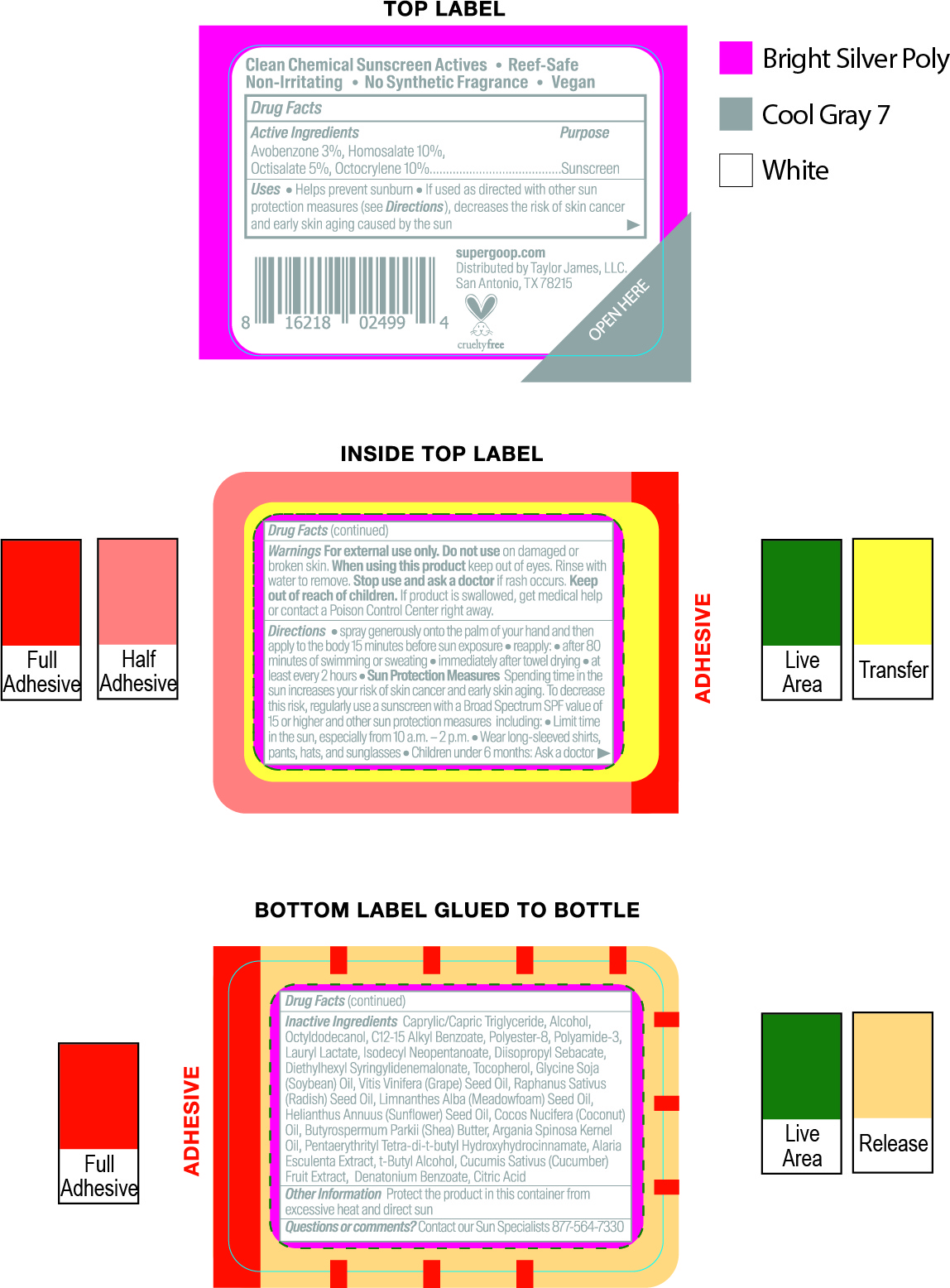
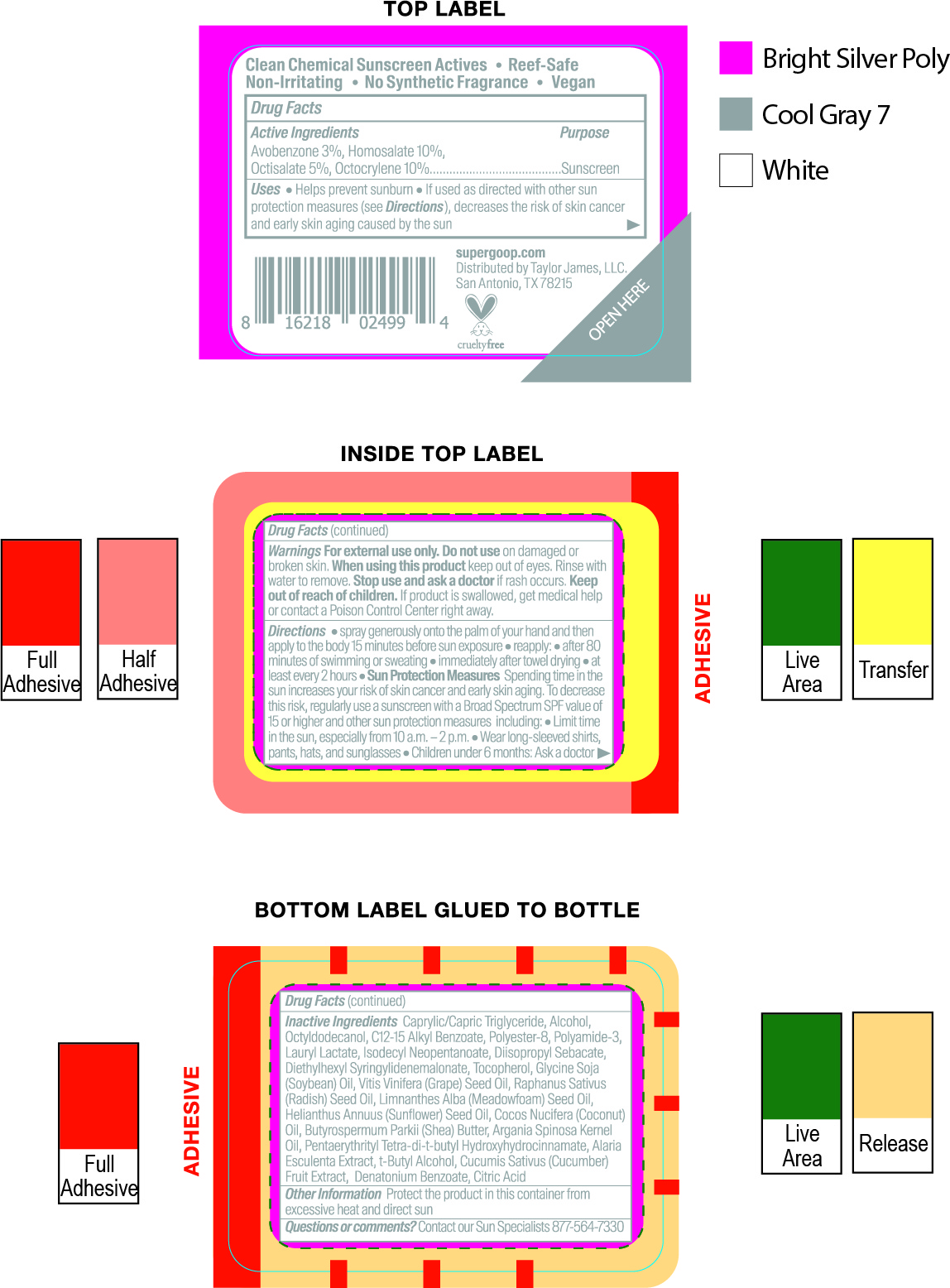
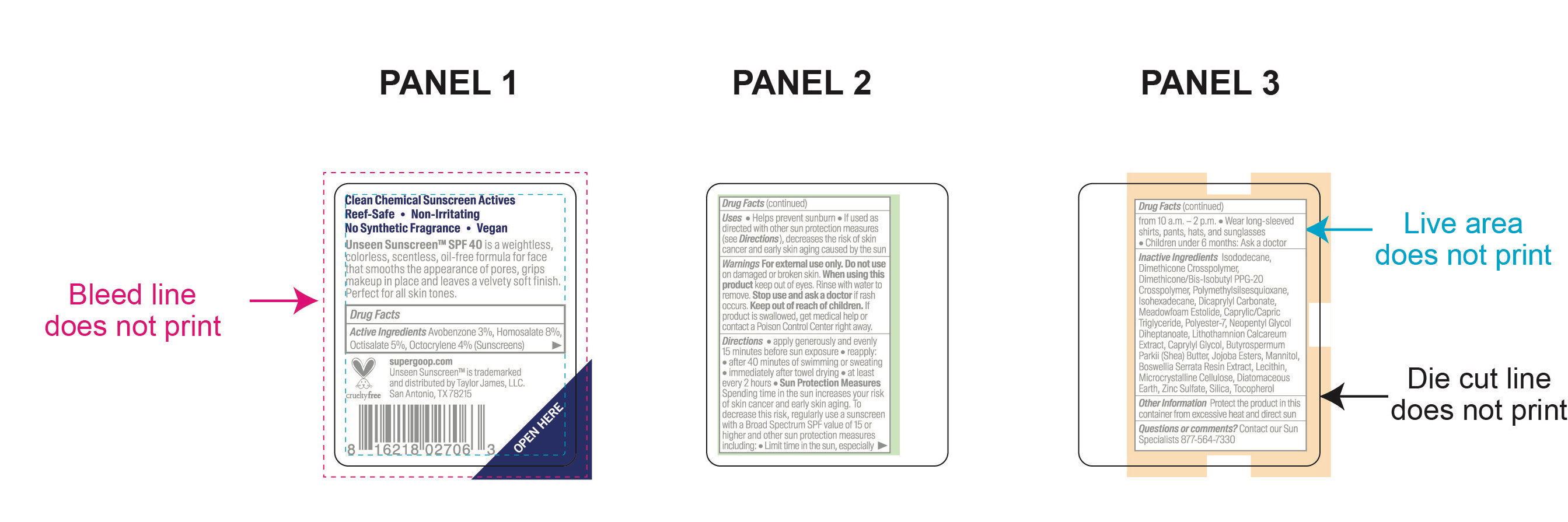
avobenzone, homosalate, octisalate, octocrylene kitProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75936-244 Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-244-01 1 in 1 KIT; Type 1: Convenience Kit of Co-Package 03/20/2021 Quantity of Parts Part # Package Quantity Total Product Quantity Part 1 1 TUBE 15 mL Part 2 1 BOTTLE, SPRAY 15 mL Part 3 2 TUBE 71 mL Part 4 1 BOTTLE 30 mL Part 5 1 BOTTLE 89 mL Part 6 1 CYLINDER 4.25 g Part 1 of 6 UNSEEN SUNSCREEN SPF 40
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Item Code (Source) NDC:75936-245 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 8 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 4 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL Inactive Ingredients Ingredient Name Strength DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) POLYMETHYLSILSESQUIOXANE (4.5 MICRONS) (UNII: 59Z907ZB69) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) ISODODECANE (UNII: A8289P68Y2) DIATOMACEOUS EARTH (UNII: 2RF6EJ0M85) ZINC SULFATE (UNII: 89DS0H96TB) DIMETHICONE CROSSPOLYMER (450000 MPA.S AT 12% IN CYCLOPENTASILOXANE) (UNII: UF7620L1W6) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) INDIAN FRANKINCENSE (UNII: 4PW41QCO2M) PHYMATOLITHON CALCAREUM (UNII: 6J1M3WA0ZK) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SHEA BUTTER (UNII: K49155WL9Y) MANNITOL (UNII: 3OWL53L36A) POLYESTER-7 (UNII: 0841698D2F) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) NEOPENTYL GLYCOL DIHEPTANOATE (UNII: 5LKW3C543X) DIMETHICONE/BIS-ISOBUTYL PPG-20 CROSSPOLYMER (UNII: O4I3UFO6ZF) ISOHEXADECANE (UNII: 918X1OUF1E) TOCOPHEROL (UNII: R0ZB2556P8) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-245-01 15 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/20/2021 Part 2 of 6 DEFENSE REFRESH (RE)SETTING MIST SPF 40
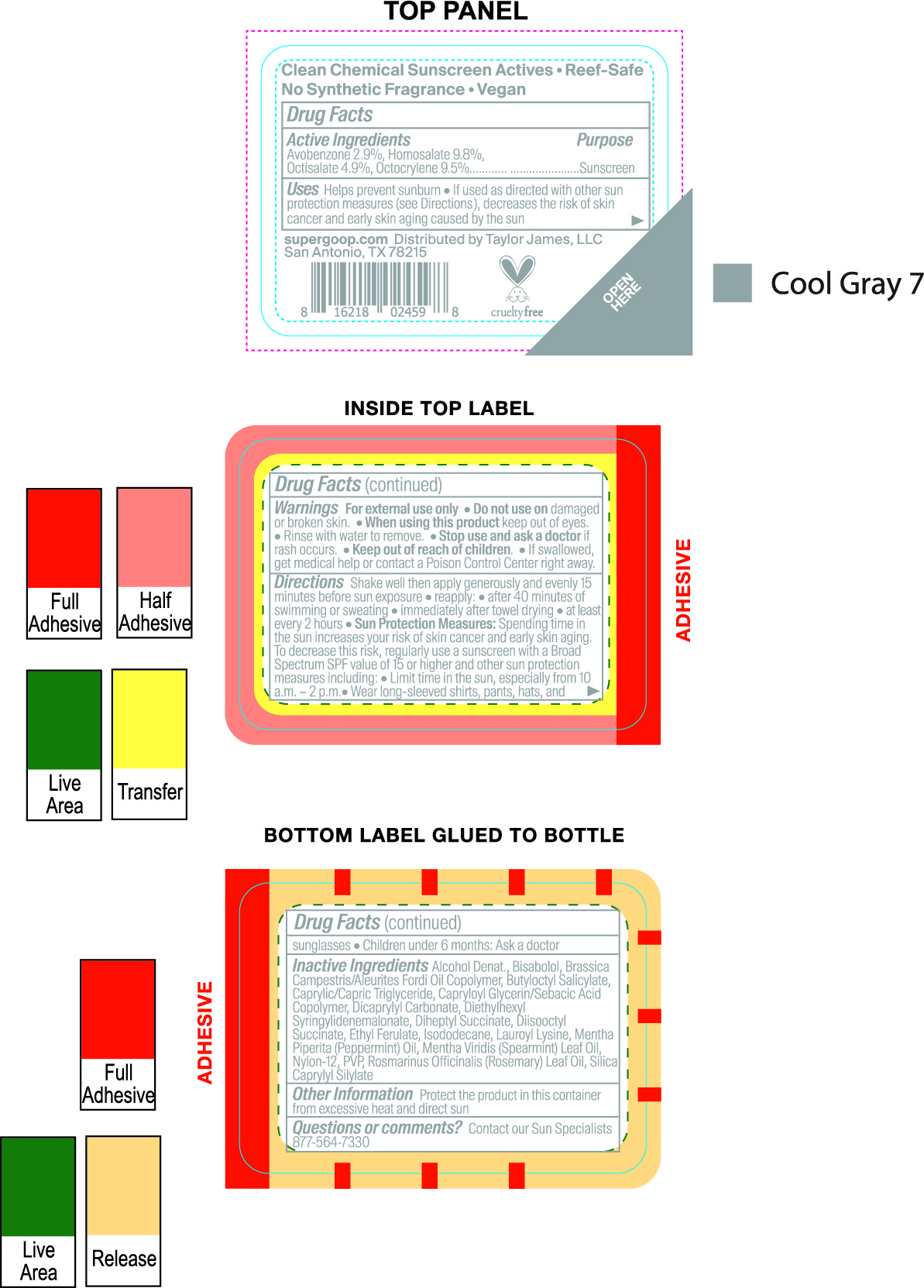
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Item Code (Source) NDC:75936-246 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.8 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9.8 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.9 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9.5 g in 100 mL Inactive Ingredients Ingredient Name Strength POVIDONE, UNSPECIFIED (UNII: FZ989GH94E) ISODODECANE (UNII: A8289P68Y2) SPEARMINT OIL (UNII: C3M81465G5) LEVOMENOL (UNII: 24WE03BX2T) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DIHEPTYL SUCCINATE (UNII: 057N7SS26Y) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) LAUROYL LYSINE (UNII: 113171Q70B) CAPRYLOYL GLYCERIN/SEBACIC ACID COPOLYMER (2000 MPA.S) (UNII: N7YC58165T) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) ALCOHOL (UNII: 3K9958V90M) ETHYL FERULATE (UNII: 5B8915UELW) PEPPERMINT OIL (UNII: AV092KU4JH) NYLON-12 (UNII: 446U8J075B) ROSEMARY OIL (UNII: 8LGU7VM393) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-246-01 30 mL in 1 BOTTLE, SPRAY; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/20/2021 Part 3 of 6 PLAY EVERYDAY SPF 50 WITH SUNFLOWER EXTRACT
avobenzone, homosalate, octisalate, octocrylene creamProduct Information Item Code (Source) NDC:75936-247 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 7.5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL Inactive Ingredients Ingredient Name Strength ISODODECANE (UNII: A8289P68Y2) CETYL ALCOHOL (UNII: 936JST6JCN) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) PELARGONIUM GRAVEOLENS FLOWER OIL (UNII: 3K0J1S7QGC) POGOSTEMON CABLIN LEAF OIL (UNII: F3IN55X5PO) PENTYLENE GLYCOL (UNII: 50C1307PZG) ALLANTOIN (UNII: 344S277G0Z) LEMON OIL, COLD PRESSED (UNII: I9GRO824LL) EUCALYPTUS OIL (UNII: 2R04ONI662) HELIANTHUS ANNUUS FLOWERING TOP (UNII: BKJ0J3D1BP) BEHENIC ACID (UNII: H390488X0A) CETYL BEHENATE (UNII: WFM51TRO3E) RICE BRAN (UNII: R60QEP13IC) CETEARYL OLIVATE (UNII: 58B69Q84JO) ROSEWOOD OIL (UNII: F2522O5L7B) CHLORPHENESIN (UNII: I670DAL4SZ) ROSEMARY (UNII: IJ67X351P9) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) SODIUM TRIPOLYPHOSPHATE ANHYDROUS (UNII: 9SW4PFD2FZ) BUTYL ACRYLATE/METHYL METHACRYLATE/METHACRYLIC ACID COPOLYMER (18000 MW) (UNII: JZ1374NL9E) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) CAPRYLYL GLYCOL (UNII: 00YIU5438U) XANTHAN GUM (UNII: TTV12P4NEE) ISOSTEARYL ISOSTEARATE (UNII: IV0Z586Z4Y) TOCOPHEROL (UNII: R0ZB2556P8) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) POTASSIUM CETYL PHOSPHATE (UNII: 03KCY6P7UT) AMMONIUM ACRYLOYLDIMETHYLTAURATE/VP COPOLYMER (UNII: W59H9296ZG) HYDROXYACETOPHENONE (UNII: G1L3HT4CMH) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) SORBITAN OLIVATE (UNII: MDL271E3GR) PANTHENOL (UNII: WV9CM0O67Z) LAURYL LACTATE (UNII: G5SU0BFK7O) ORANGE OIL, COLD PRESSED (UNII: AKN3KSD11B) OCIMUM BASILICUM FLOWERING TOP (UNII: 7SAB275FP2) WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) TRISODIUM ETHYLENEDIAMINE DISUCCINATE (UNII: YA22H34H9Q) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-247-01 30 mL in 1 TUBE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/20/2021 Part 4 of 6 GLOW OIL SUNSCREEN SPF 50
avobenzone, homosalate, octisalate, octocrylene oilProduct Information Item Code (Source) NDC:75936-248 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL Inactive Ingredients Ingredient Name Strength ALKYL (C12-15) BENZOATE (UNII: A9EJ3J61HQ) DENATONIUM BENZOATE ANHYDROUS (UNII: M5BA6GAF1O) PENTAERYTHRITOL TETRAKIS(3-(3,5-DI-TERT-BUTYL-4-HYDROXYPHENYL)PROPIONATE) (UNII: 255PIF62MS) DIETHYLHEXYL SYRINGYLIDENEMALONATE (UNII: 3V5U97P248) LAURYL LACTATE (UNII: G5SU0BFK7O) CUCUMBER (UNII: YY7C30VXJT) ALCOHOL (UNII: 3K9958V90M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) TOCOPHEROL (UNII: R0ZB2556P8) SOYBEAN OIL (UNII: 241ATL177A) TERT-BUTYL ALCOHOL (UNII: MD83SFE959) ARGAN OIL (UNII: 4V59G5UW9X) COCONUT OIL (UNII: Q9L0O73W7L) SHEA BUTTER (UNII: K49155WL9Y) POLYAMIDE-3 (12000 MW) (UNII: L7P3YWF22X) MEADOWFOAM SEED OIL (UNII: 412ZHA4T4Y) SUNFLOWER OIL (UNII: 3W1JG795YI) GRAPE SEED OIL (UNII: 930MLC8XGG) ALARIA ESCULENTA (UNII: EJ9JK8J58D) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) DIISOPROPYL SEBACATE (UNII: J8T3X564IH) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-248-01 30 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/08/2021 Part 5 of 6 PLAY ANTIOXIDANT BODY MIST SPF 50 WITH VITAMIN C
avobenzone, homosalate, octisalate, octocrylene sprayProduct Information Item Code (Source) NDC:75936-249 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 4.9 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 9.8 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9.5 g in 100 mL AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 2.8 g in 100 mL Inactive Ingredients Ingredient Name Strength ORANGE OIL TERPENELESS (UNII: L8B7EWV9I7) GREEN TEA LEAF (UNII: W2ZU1RY8B0) CANANGA OIL (UNII: 8YOY78GNNX) LIME OIL (UNII: UZH29XGA8G) CITRUS MAXIMA FRUIT RIND OIL (UNII: 8U3877WD44) CITRUS RETICULATA LEAF OIL (UNII: 1515UE78IH) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) DICAPRYLYL CARBONATE (UNII: 609A3V1SUA) DIISOPROPYL ADIPATE (UNII: P7E6YFV72X) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) LEMON OIL (UNII: I9GRO824LL) ORANGE OIL (UNII: AKN3KSD11B) ACAI (UNII: 46AM2VJ0AW) LAVENDER OIL (UNII: ZBP1YXW0H8) PEPPERMINT OIL (UNII: AV092KU4JH) SPEARMINT OIL (UNII: C3M81465G5) GRAPE SEED OIL (UNII: 930MLC8XGG) ETHYL FERULATE (UNII: 5B8915UELW) ALCOHOL (UNII: 3K9958V90M) ISODECYL NEOPENTANOATE (UNII: W60VYE24XC) SUNFLOWER OIL (UNII: 3W1JG795YI) TETRAHEXYLDECYL ASCORBATE (UNII: 9LBV3F07AZ) TRIDECYL NEOPENTANOATE (UNII: 3Z8H1DA7J5) MANDARIN OIL (UNII: NJO720F72R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-249-01 89 mL in 1 BOTTLE; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/14/2021 Part 6 of 6 PLAY LIP SHIELD WITH MINT BROAD SPECTRUM SPF 30
avobenzone, homosalate, octisalate, octocrylene stickProduct Information Item Code (Source) NDC:75936-253 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 g AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 g OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 g OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 10 g in 100 g Inactive Ingredients Ingredient Name Strength SHEA BUTTER (UNII: K49155WL9Y) STEVIA REBAUDIUNA LEAF (UNII: 6TC6NN0876) GRAPE SEED OIL (UNII: 930MLC8XGG) ALOE VERA LEAF (UNII: ZY81Z83H0X) TOCOPHEROL (UNII: R0ZB2556P8) COCONUT OIL (UNII: Q9L0O73W7L) CERESIN (UNII: Q1LS2UJO3A) CANDELILLA WAX (UNII: WL0328HX19) SUNFLOWER OIL (UNII: 3W1JG795YI) BUTYLOCTYL SALICYLATE (UNII: 2EH13UN8D3) JOJOBA OIL (UNII: 724GKU717M) AVOCADO OIL (UNII: 6VNO72PFC1) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75936-253-01 4.25 g in 1 CYLINDER; Type 1: Convenience Kit of Co-Package Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 04/26/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part352 03/20/2021 Labeler - TAYLOR JAMES, LTD. (033381850)