Label: SAN SKINLAB CLOTH WET WIPES- benzalkonium chloride 0.13% cloth
- NDC Code(s): 75214-120-01
- Packager: HANUL CO.,LTD
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 9, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
SAN SKINLAB CLOTH WET WIPES
benzalkonium chloride 0.13% clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75214-120 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 mg in 100 mL Inactive Ingredients Ingredient Name Strength ALCOHOL (UNII: 3K9958V90M) CITRIC ACID MONOHYDRATE (UNII: 2968PHW8QP) 1,2-HEXANEDIOL (UNII: TR046Y3K1G) ALOE VERA LEAF (UNII: ZY81Z83H0X) GLYCERIN (UNII: PDC6A3C0OX) BUTYLENE GLYCOL (UNII: 3XUS85K0RA) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75214-120-01 798 mL in 1 CANISTER; Type 0: Not a Combination Product 04/12/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 04/12/2021 Labeler - HANUL CO.,LTD (557814370) Establishment Name Address ID/FEI Business Operations HANUL CO., LTD 557814370 manufacture(75214-120)


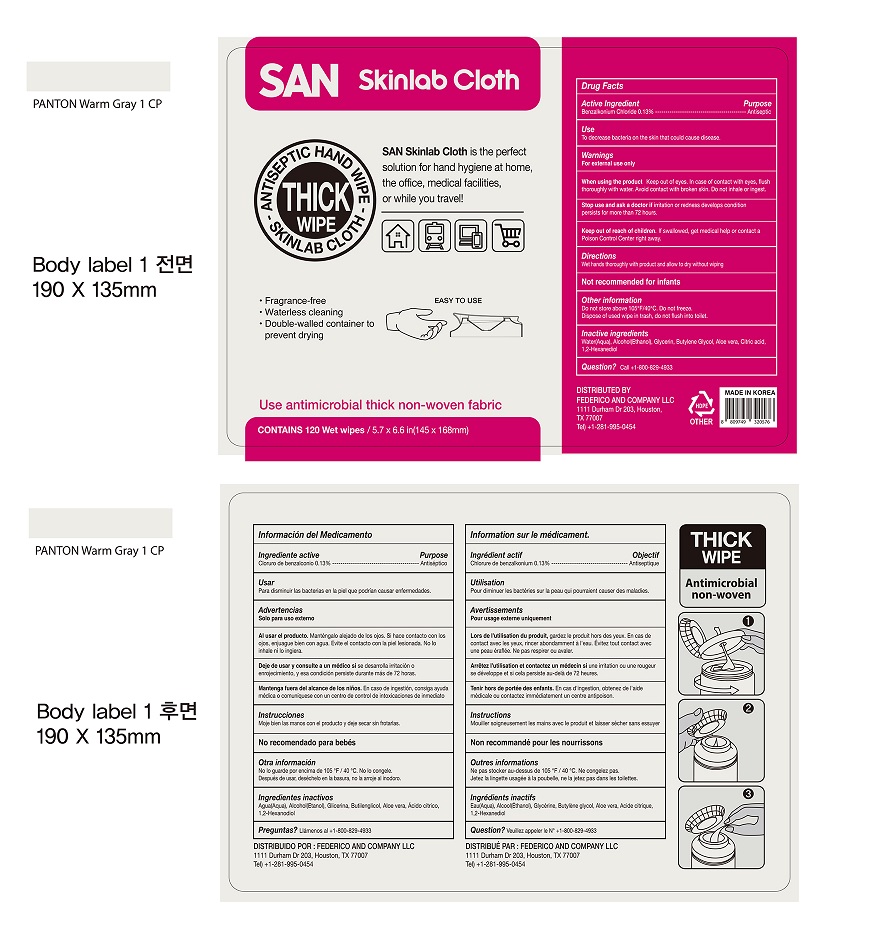 120 Wet wipes NDC 75214-120-01
120 Wet wipes NDC 75214-120-01