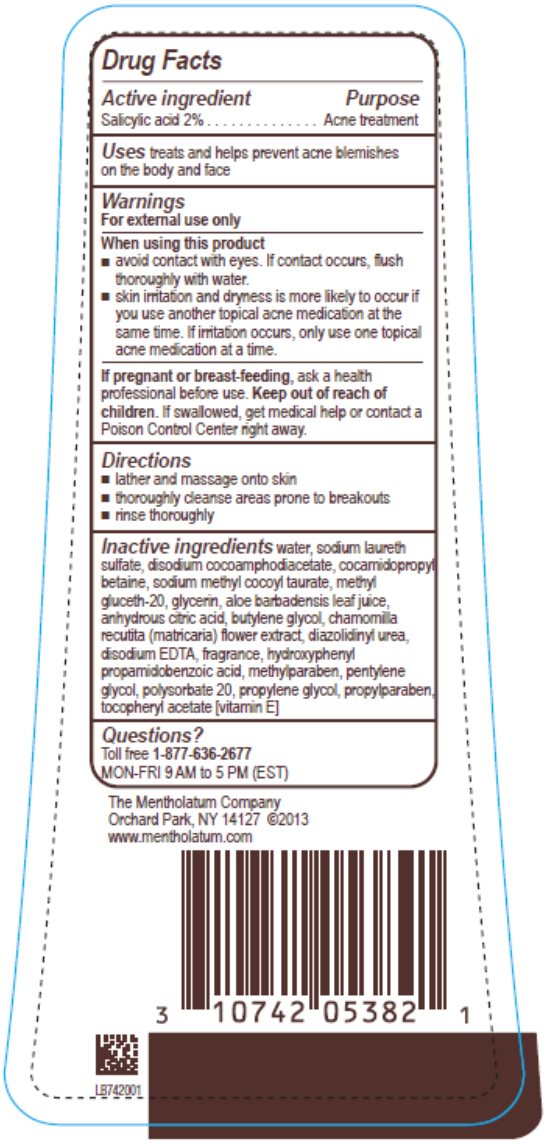
Label: PHISODERM ANTI BLEMISH BODY WASH- salicylic acid liquid
- NDC Code(s): 10742-8451-1
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 17, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
- Directions
-
Inactive ingredients
water, sodium laureth sulfate, disodium cocoamphodiacetate, cocamidopropyl betaine, sodium methyl cocoyl taurate, methyl gluceth-20, glycerin, aloe barbadensis leaf juice, anhydrous citric acid, butylene glycol, chamomilla recutita (matricaria) flower extract, diazolidinyl urea, disodium EDTA, fragrance, hydroxyphenyl propamidobenzoic acid, methylparaben, pentylene glycol, polysorbate 20, propylene glycol, propylparaben, tocopheryl acetate [vitamin E]
- Questions?
- Principal Display Panel
- Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PHISODERM ANTI BLEMISH BODY WASH
salicylic acid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-8451 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM LAURETH-3 SULFATE (UNII: BPV390UAP0) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) COCAMIDOPROPYL BETAINE (UNII: 5OCF3O11KX) SODIUM METHYL COCOYL TAURATE (UNII: JVL98CG53G) METHYL GLUCETH-20 (UNII: J3QD0LD11P) GLYCERIN (UNII: PDC6A3C0OX) ALOE VERA LEAF (UNII: ZY81Z83H0X) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) 1,2-BUTANEDIOL (UNII: RUN0H01QEU) CHAMOMILE (UNII: FGL3685T2X) DIAZOLIDINYL UREA (UNII: H5RIZ3MPW4) EDETATE DISODIUM (UNII: 7FLD91C86K) HYDROXYPHENYL PROPAMIDOBENZOIC ACID (UNII: 25KRT26H77) METHYLPARABEN (UNII: A2I8C7HI9T) PENTYLENE GLYCOL (UNII: 50C1307PZG) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PROPYLPARABEN (UNII: Z8IX2SC1OH) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-8451-1 295 mL in 1 BOTTLE; Type 0: Not a Combination Product 09/01/2013 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/01/2013 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-8451)