Label: ALCOHOL WET WIPE gel
HAND SANITIZER- hand sanitizer gel gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 54237-030-01, 54237-030-02, 54237-030-03, 54237-030-04, view more54237-030-05, 54237-030-06, 54237-030-07, 54237-030-08, 54237-030-09, 54237-030-10, 54237-030-11, 54237-030-12, 54237-030-13, 54237-031-01, 54237-031-02, 54237-031-03, 54237-031-04, 54237-031-05, 54237-031-06, 54237-031-07, 54237-031-08, 54237-031-09 - Packager: Guangzhou Daieme Cosmetic Co,.Ltd
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 2, 2021
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Main active ingredient
-
SPL UNCLASSIFIED SECTION
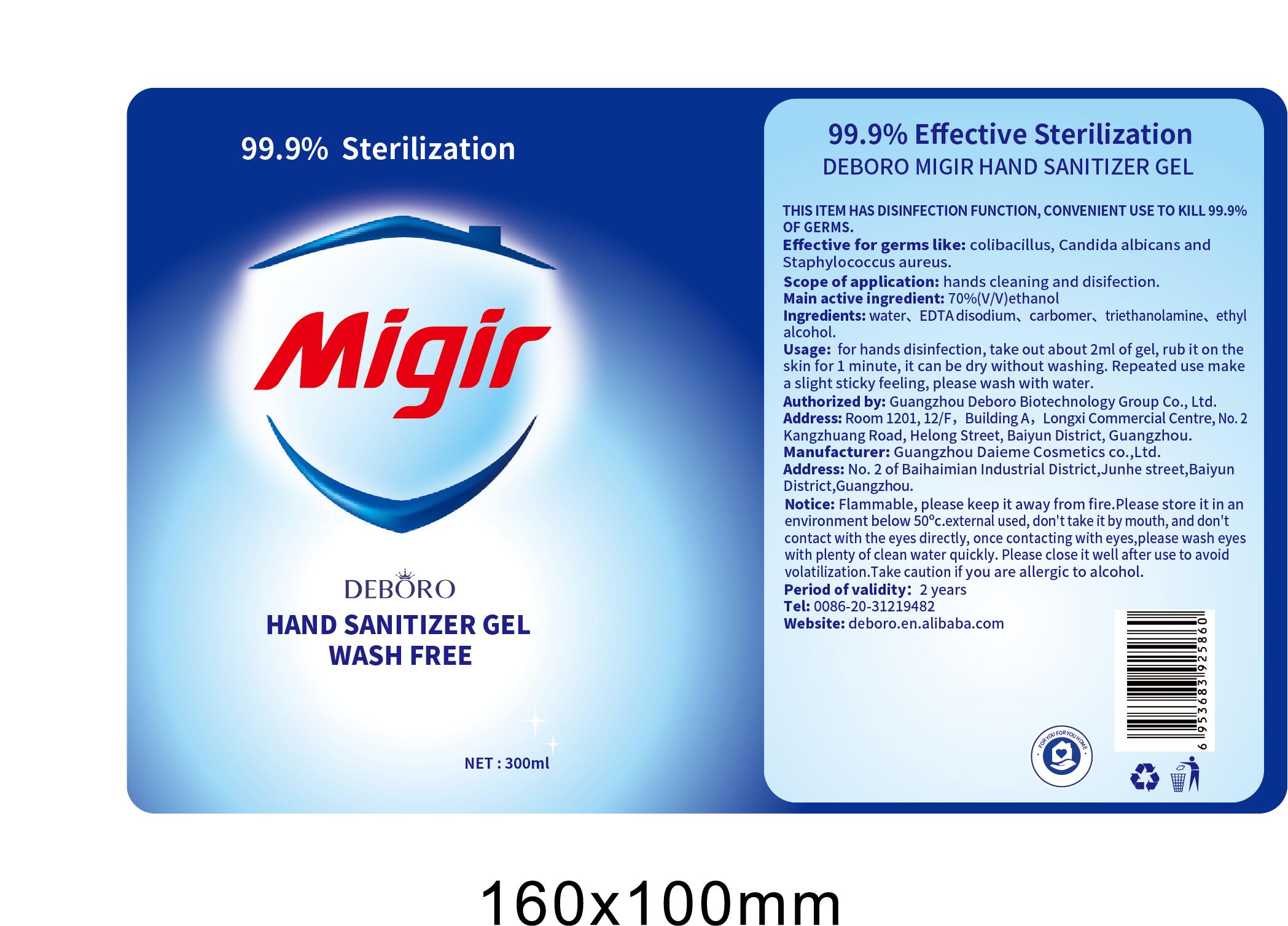
- THIS ITEM HAS DISINFECTION FUNCTION, CONVENIENT USE TO KILL 99.9% OF GERMS.
- Effective for germs like: colibasillus. Candida albicans anc Staphylococcus aureus.
- Scope of application: hands cleaning and disifection.
- Main active ingredient: 70%(V/V)ethanol
- Ingredients: water, EDTA disodium, carbomer, triethanolamine, ethyalcohol.
- Usage: for hands disinfection, take out about 2ml of gel, rub it on the skin for 1 minute, it can be dry without washing. Repeated use make a slight sticky feeling, please wash with water.
- Notice: Flammable, please keep it away from fire.Please store it in an environment below 50℃.external used, don't take it by mouth, and don't contact with the eyes directly, once contacting with eyes,please wash eyes with plenty of clean water quickly. Please close it well after use to avoid volatilization.Take caution if you are allergic to alcohol.
- Period of validity: 2 years
- Scope of application
- Usage
-
Notice
Flammable, please keep it away from fire.Please store it in an environment below 50℃.external used, don'ttake it by mouth, and don't contact with the eyes directly, once contacting with eyes,please wash eyes with plenty of clean water quickly. Please close it well after use to avoid volatilization.Take caution if you are allergic to alcohol.
- Do not use
-
WHEN USING
Flammable, please keep it away from fire.Please store it in an environment below 50°c.external used, don'ttake it by mouth, and don't contact with the eyes directly, once contacting with eyes,please wash eyes with plenty of clean water quickly. Please close it well after use to avoid volatilization.Take caution if you are allergic to alcohol.
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- ingredients
- Package label
-
INGREDIENTS AND APPEARANCE
ALCOHOL WET WIPE
alcohol wet wipe gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54237-031 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 72.7 mL in 100 mL Inactive Ingredients Ingredient Name Strength POLYAMINOPROPYL BIGUANIDE (UNII: DT9D8Z79ET) 0.5 mL in 100 mL GLYCERIN (UNII: PDC6A3C0OX) 1 mL in 100 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54237-031-01 1 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 2 NDC:54237-031-02 8 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 3 NDC:54237-031-03 10 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 4 NDC:54237-031-04 20 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 5 NDC:54237-031-05 25 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 6 NDC:54237-031-06 48 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 7 NDC:54237-031-07 50 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 8 NDC:54237-031-08 80 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 9 NDC:54237-031-09 100 mL in 1 BAG; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/30/2020 HAND SANITIZER
hand sanitizer gel gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:54237-030 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength TROLAMINE (UNII: 9O3K93S3TK) 0.28 mL in 100 mL EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) 0.01 mL in 100 mL CARBOMER COPOLYMER TYPE A (UNII: 71DD5V995L) 0.28 mL in 100 mL WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:54237-030-01 2 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 2 NDC:54237-030-02 30 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 3 NDC:54237-030-03 60 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 4 NDC:54237-030-04 99 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 5 NDC:54237-030-05 100 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 6 NDC:54237-030-06 120 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 7 NDC:54237-030-07 300 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 8 NDC:54237-030-08 500 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 9 NDC:54237-030-09 1000 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 10 NDC:54237-030-10 29.57 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 11 NDC:54237-030-11 118.28 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 12 NDC:54237-030-12 236.56 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 13 NDC:54237-030-13 473.12 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 03/30/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 03/30/2020 Labeler - Guangzhou Daieme Cosmetic Co,.Ltd (542359812) Establishment Name Address ID/FEI Business Operations Guangzhou Daieme Cosmetic Co,.Ltd 542359812 manufacture(54237-030, 54237-031)