Label: PLANEAIRE HANDS- ethanol hand sanitizer gel
- NDC Code(s): 80335-112-02
- Packager: EverywhereAire LLC
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 20, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient(s)
- Purpose
- Use
- Warnings
- Do not use
-
WHEN USING
When using this product keep out of eyes. In case of contact with eyes, rinse eyes thoroughly with water.
Stop use if irritation or rash occurs. If condition persists for more than 72 hours, consult a doctor.
Keep out of reach of children. If swallowed, get medical help or contact a Poison Control Center right away. - STOP USE
- KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
PLANEAIRE HANDS
ethanol hand sanitizer gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:80335-112 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 70 mL in 100 mL Inactive Ingredients Ingredient Name Strength GREEN TEA LEAF (UNII: W2ZU1RY8B0) PEPPERMINT OIL (UNII: AV092KU4JH) SODIUM HYDROXIDE (UNII: 55X04QC32I) ALOE VERA LEAF POLYSACCHARIDES (UNII: W21O437517) SPEARMINT (UNII: J7I2T6IV1N) MINT (UNII: FV98Z8GITP) GLYCERIN (UNII: PDC6A3C0OX) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:80335-112-02 60 mL in 1 BOTTLE, PUMP; Type 0: Not a Combination Product 01/11/2021 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 01/11/2021 Labeler - EverywhereAire LLC (117635273) Registrant - EverywhereAire LLC (117635273)

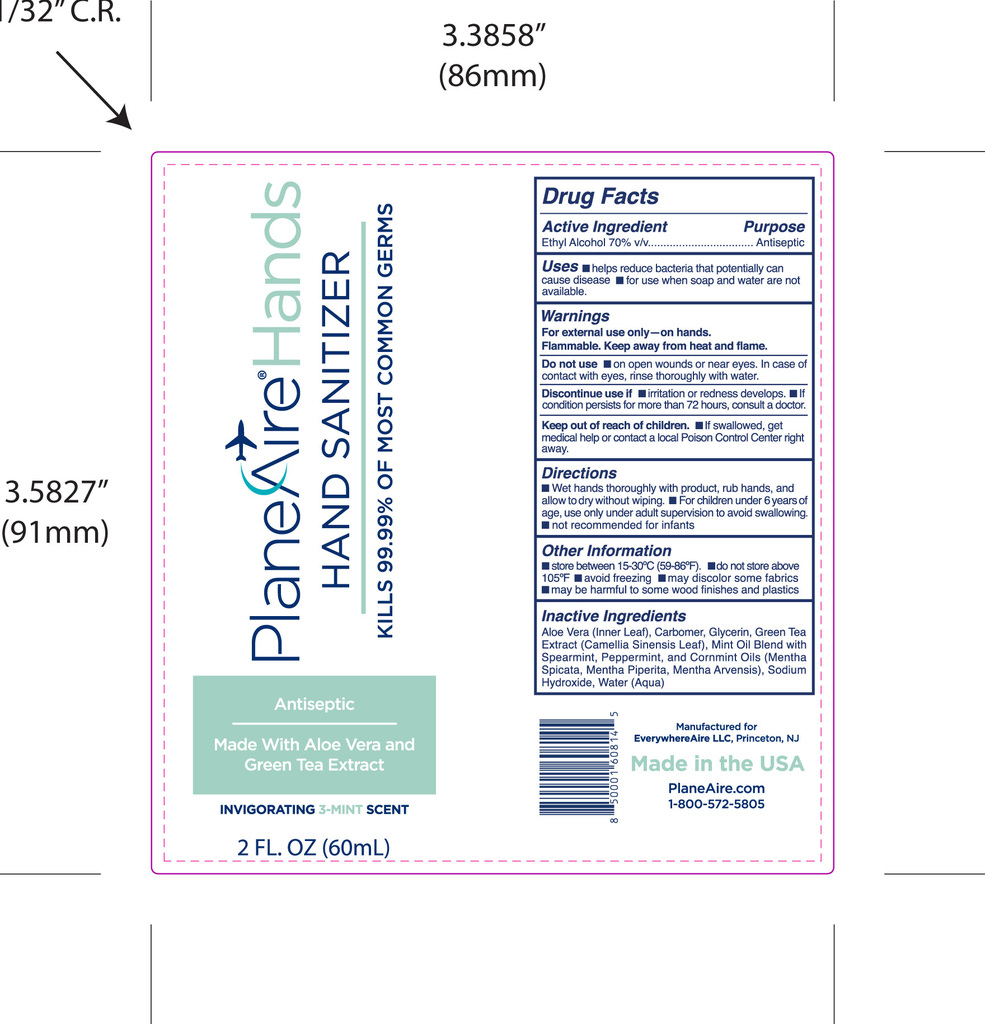
 60 mL NDC: 80335-112-02
60 mL NDC: 80335-112-02