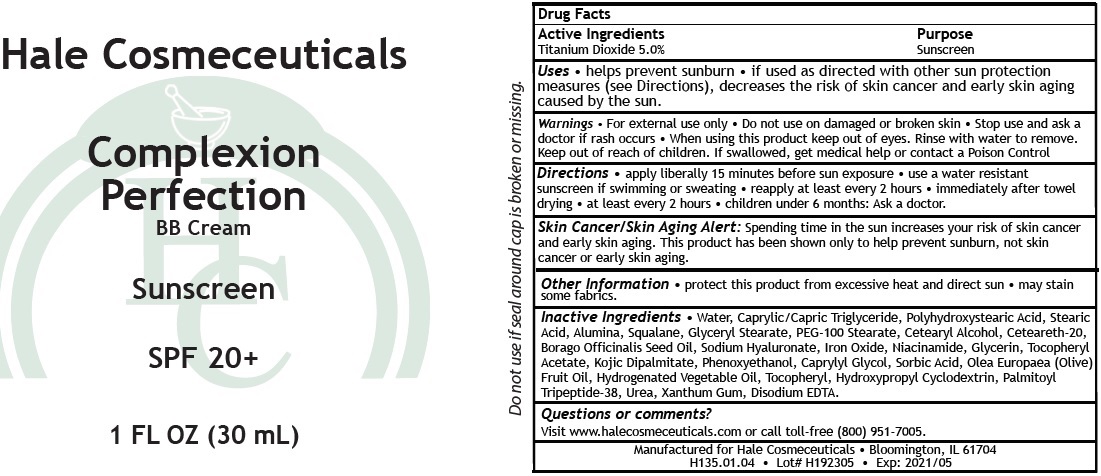
Label: HALE COSMECEUTICALS COMPLEXION PERFECTION SUNSCREEN SPF 20- titanium dioxide cream
- NDC Code(s): 71987-007-01
- Packager: Hale Cosmeceuticals, Inc.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated December 14, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active Ingredients
- Uses
- Warnings
-
Directions
- apply liberally 15 minutes before sun exposure
- use a water resistant sunscreen if swimming or sweating
- reapply at least every 2 hours
- immediately after towel drying
- at least every 2 hours
- children under 6 months: Ask a doctor
Skin Cancer/Skin Aging Alert: Spending time in the sun increases your risk of skin cancer and early skin aging. This product has been shown only to help prevent sunburn, not skin cancer or early skin aging.
- Other Information
-
Inactive Ingredients
- Water, Caprylic/Capric Triglyceride, Polyhydroxystearic Acid, Stearic Acid, Alumina, Squalane, Glyceryl Stearate, PEG-100 Stearate, Cetearyl Alcohol, Ceteareth-20, Borago Officinalis Seed Oil, Sodium Hyaluronate, Iron Oxide, Niacinamide, Glycerin, Tocopheryl Acetate, Kojic Dipalmitate, Phenoxyethanol, Caprylyl Glycol, Sorbic Acid, Olea Europaea (Olive) Fruit Oil, Hydrogenated Vegetable Oil, Tocopheryl, Hydroxypropyl Cyclodextrin, Palmitoyl Tripeptide-38, Urea, Xanthum Gum, Disodium EDTA.
- Questions or comments?
- Package Labeling:
-
INGREDIENTS AND APPEARANCE
HALE COSMECEUTICALS COMPLEXION PERFECTION SUNSCREEN SPF 20
titanium dioxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:71987-007 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength TITANIUM DIOXIDE (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) TITANIUM DIOXIDE 50 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) MEDIUM-CHAIN TRIGLYCERIDES (UNII: C9H2L21V7U) POLYHYDROXYSTEARIC ACID (2300 MW) (UNII: YXH47AOU0F) STEARIC ACID (UNII: 4ELV7Z65AP) ALUMINUM OXIDE (UNII: LMI26O6933) SQUALANE (UNII: GW89575KF9) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) PEG-100 STEARATE (UNII: YD01N1999R) CETOSTEARYL ALCOHOL (UNII: 2DMT128M1S) POLYOXYL 20 CETOSTEARYL ETHER (UNII: YRC528SWUY) BORAGO OFFICINALIS SEED (UNII: 2GXJ790US0) HYALURONATE SODIUM (UNII: YSE9PPT4TH) FERRIC OXIDE RED (UNII: 1K09F3G675) NIACINAMIDE (UNII: 25X51I8RD4) GLYCERIN (UNII: PDC6A3C0OX) .ALPHA.-TOCOPHEROL ACETATE (UNII: 9E8X80D2L0) KOJIC DIPALMITATE (UNII: 13N249RWTM) PHENOXYETHANOL (UNII: HIE492ZZ3T) CAPRYLYL GLYCOL (UNII: 00YIU5438U) SORBIC ACID (UNII: X045WJ989B) OLIVE OIL (UNII: 6UYK2W1W1E) TOCOPHEROL (UNII: R0ZB2556P8) PALMITOYL LYSYLDIOXYMETHIONYLLYSINE (UNII: T7A529FB8O) UREA (UNII: 8W8T17847W) XANTHAN GUM (UNII: TTV12P4NEE) EDETATE DISODIUM ANHYDROUS (UNII: 8NLQ36F6MM) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:71987-007-01 30 mL in 1 TUBE; Type 0: Not a Combination Product 12/01/2020 12/31/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 12/01/2020 12/31/2024 Labeler - Hale Cosmeceuticals, Inc. (168405822)