Label: DRIZALMA SPRINKLE- duloxetine capsule, delayed release
-
NDC Code(s):
47335-616-10,
47335-616-30,
47335-616-60,
47335-616-90, view more47335-617-10, 47335-617-30, 47335-617-60, 47335-617-90, 47335-618-10, 47335-618-30, 47335-618-60, 47335-618-90, 47335-619-10, 47335-619-30, 47335-619-60, 47335-619-90
- Packager: SUN PHARMACEUTICAL INDUSTRIES, INC.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 14, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Medication Guide: HTML
- Official Label (Printer Friendly)
-
HIGHLIGHTS OF PRESCRIBING INFORMATION
These highlights do not include all the information needed to use DRIZALMA SPRINKLE safely and effectively. See full prescribing information for DRIZALMA SPRINKLE.
DRIZALMA SPRINKLE™ (duloxetine delayed-release capsules), for oral use.
Initial U.S. Approval: 2004WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
See full prescribing information for complete boxed warning.
•Increased risk of suicidal thinking and behavior in pediatric and young adult patients taking antidepressants (5.1)
•Closely monitor all antidepressant-treated patients for clinical worsening and emergence of suicidal thoughts and behaviors (5.1)
RECENT MAJOR CHANGES
Warnings and Precautions (5.4, 5.5) 08/2023
INDICATIONS AND USAGE
DRIZALMA SPRINKLE is a serotonin and norepinephrine reuptake inhibitor (SNRI) indicated for the treatment of following conditions:
• Major Depressive Disorder (MDD) in adults (1)
• Generalized Anxiety Disorder (GAD) in adults and pediatric patients ages 7 years of age and older (1)
• Diabetic Peripheral Neuropathic Pain (DPNP) in adults (1)
• Fibromyalgia (FM) in adults (1)
• Chronic Musculoskeletal Pain in adults (1) (1)DOSAGE AND ADMINISTRATION
- •
- Take DRIZALMA SPRINKLE with or without food (2.1)
- •
- DRIZALMA SPRINKLE may be: swallowed whole (do not crush or chew capsule); opened and sprinkled over applesauce; or administered via nasogastric tube (2.1)
- •
- Take a missed dose as soon as it is remembered. Do not take two doses of DRIZALMA SPRINKLE at the same time (2.1)
Indication (2)
Starting Dose (2)
Target Dose (2)
Maximum Dose (2)
MDD (2.2) (2)
Adults (2)
40 mg/day to (2)
60 mg/day (2)
Acute Treatment: 40 mg/day (20 mg twice daily) to 60 mg/day (once daily or as 30 mg twice daily); Maintenance Treatment: 60 mg/day (2)
120 mg/day (2)
GAD (2.3) (2)
Adults (2)
60 mg/day (2)
60 mg/day (once daily) (2)
120 mg/day (2)
Geriatric (2)
30 mg/day (2)
60 mg/day (once daily) (2)
120 mg/day (2)
Pediatrics (7 to 17 years of age) (2)
30 mg/day (2)
30 to 60 mg/day (once daily) (2)
120 mg/day (2)
DPNP (2.4) (2)
60 mg/day (2)
60 mg/day (once daily) (2)
60 mg/day (2)
FM (2.5) (2)
Adults (2)
30 mg/day (2)
60 mg/day (once daily) (2)
60 mg/day (2)
Chronic Musculoskeletal Pain (2)
(2.6) Adults (2)
30 mg/day (2)
60 mg/day (once daily) (2)
60 mg/day (2)
- •
- There is no evidence that doses greater than 60 mg/day confers additional benefit, while some adverse reactions were observed to be dose-dependent (2)
- •
- Discontinuing DRIZALMA SPRINKLE: Gradually reduce dosage to avoid discontinuation symptoms (2.8, 5.7)
DOSAGE FORMS AND STRENGTHS
Delayed-release capsules: 20 mg, 30 mg, 40 mg, and 60 mg (3) (3)
CONTRAINDICATIONS
- Serotonin Syndrome and MAOIs: Do not use MAOIs intended to treat psychiatric disorders with DRIZALMA SPRINKLE or within 5 days of stopping treatment with DRIZALMA SPRINKLE. Do not use DRIZALMA SPRINKLE within 14 days of stopping an MAOI intended to treat psychiatric disorders. In addition, do not start DRIZALMA SPRINKLE in a patient who is being treated with linezolid or intravenous methylene blue (4)
WARNINGS AND PRECAUTIONS
-
Hepatotoxicity: Hepatic failure, sometimes fatal, has been reported. Discontinue DRIZALMA SPRINKLE in patients who develop jaundice or other evidence of clinically significant liver dysfunction and should not be resumed unless another cause can be established. Avoid use in patients with substantial alcohol use or evidence of chronic liver disease (5.2)
- Orthostatic Hypotension, Falls and Syncope: Consider dosage reduction or discontinuation if these events occur (5.3)
- Serotonin Syndrome: Increased risk when co-administered with other serotonergic agents, but also when taken alone. If it occurs, discontinue DRIZALMA SPRINKLE and serotonergic agents and initiate supportive treatment (5.4)
- Increased Risk of Bleeding: May increase the risk of bleeding events. Concomitant use of antiplatelet drugs and anticoagulants may increase this risk (5.5, 7, 8.1)
- Severe Skin Reactions: Severe skin reactions, including erythema multiforme and Stevens-Johnson Syndrome (SJS), can occur with duloxetine. Discontinue at the first appearance of blisters, peeling rash, mucosal erosions, or any other sign of hypersensitivity if no other etiology can be identified (5.6)
- Discontinuation Syndrome: Taper dose when possible and monitor for discontinuation symptoms (5.7)
- Activation of Mania or Hypomania: Prior to initiating, screen patients for personal or family history of bipolar disorder, mania, or hypomania (5.8)
- Angle-Closure Glaucoma: Has occurred in patients with untreated anatomically narrow angles treated with antidepressants (5.9)
- Seizures: Prescribe with care in patients with a history of seizure disorder (5.10)
- Blood Pressure Increases: Monitor blood pressure prior to initiating treatment and periodically throughout treatment (5.11)
-
Inhibitors of CYP1A2 or Thioridazine: Avoid co-administration with DRIZALMA SPRINKLE (5.12)
- Hyponatremia: Can occur in association with SIADH; consider discontinuation (5.13)
- Glucose Control in Diabetes: In DPNP patients, increase in fasting blood glucose, and HbA1c have been observed (5.14)
- Conditions that Slow Gastric Emptying: Use cautiously in these patients (5.14)
- Sexual Dysfunction: DRIZALMA SPRINKLE may cause symptoms of sexual dysfunction (5.16)
ADVERSE REACTIONS
Most common adverse reactions (≥5% and at least twice the incidence of placebo-treated patients): (6.1) (6)
Adults: nausea, dry mouth, somnolence, constipation, decreased appetite, and hyperhidrosis (6)
Pediatric Patients: nausea, diarrhea, decreased weight (6)
To report SUSPECTED ADVERSE REACTIONS, contact Sun Pharmaceutical Industries, Inc. at 1-800-818-4555 or FDA at 1-800-FDA-1088 or www.fda.gov/medwatch. (6)
DRUG INTERACTIONS
- •
- Potent CYP1A2 Inhibitors: Avoid concomitant use (2.6, 7)
- •
- CYP2D6 Substrates: Consider dose reduction with concomitant use (7)
USE IN SPECIFIC POPULATIONS
- •
- Pregnancy: Third trimester use may increase risk for symptoms of poor adaptation (respiratory distress, temperature instability, feeding difficulty, hypotonia, tremor, irritability) in the neonate (8.1)
- •
- Hepatic Impairment: Avoid use in patients with chronic liver disease or cirrhosis (5.14)
- •
- Renal Impairment: Avoid use in patients with severe renal impairment, GFR <30 mL/minute (5.14)
See 17 for PATIENT COUNSELING INFORMATION and Medication Guide.
Revised: 5/2024
-
Table of Contents
FULL PRESCRIBING INFORMATION: CONTENTS*
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
1 INDICATIONS AND USAGE
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
2.2 Dosage for Treatment of Major Depressive Disorder in Adults
2.3 Dosage for Treatment of Generalized Anxiety Disorder
2.4 Dosage for Treatment of Diabetic Peripheral Neuropathic Pain in Adults
2.5 Dosage for Treatment of Fibromyalgia in Adults
2.6 Dosage for Treatment of Chronic Musculoskeletal Pain in Adults
2.7 Dosage Recommendations for Concomitant Use with Potent CYP1A2 Inhibitors
2.8 Dosage in Patients with Hepatic Impairment or Severe Renal Impairment
2.9 Screen for Bipolar Disorder Prior to Starting DRIZALMA SPRINKLE
2.10 Discontinuing DRIZALMA SPRINKLE
2.11 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
2.12 Use of DRIZALMA SPRINKLE with Other MAOIs such as Linezolid or Methylene Blue
3 DOSAGE FORMS AND STRENGTHS
4 CONTRAINDICATIONS
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
5.2 Hepatotoxicity
5.3 Orthostatic Hypotension, Falls and Syncope
5.4 Serotonin Syndrome
5.5 Increased Risk of Bleeding
5.6 Severe Skin Reactions
5.7 Discontinuation Syndrome
5.8 Activation of Mania/Hypomania
5.9 Angle-Closure Glaucoma
5.10 Seizures
5.11 Increases in Blood Pressure
5.12 Clinically Important Drug Interactions
5.13 Hyponatremia
5.14 Use in Patients with Concomitant Illness
5.15 Urinary Hesitation and Retention
5.16 Sexual Dysfunction
6 ADVERSE REACTIONS
6.1 Clinical Trials Experience
6.2 Postmarketing Experience
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with DRIZALMA SPRINKLE
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
8.2 Lactation
8.4 Pediatric Use
8.5 Geriatric Use
8.6 Gender
8.7 Smoking Status
8.8 Race
8.9 Hepatic Impairment
8.10 Severe Renal Impairment
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
9.3 Dependence
10 OVERDOSAGE
10.1 Signs and Symptoms
10.2 Management of Overdose
11 DESCRIPTION
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
12.2 Pharmacodynamics
12.3 Pharmacokinetics
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
14 CLINICAL STUDIES
14.1 Major Depressive Disorder
14.2 Generalized Anxiety Disorder
14.3 Diabetic Peripheral Neuropathic Pain in Adults
14.4 Fibromyalgia
14.5 Chronic Musculoskeletal Pain in Adults
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
undefined
17 PATIENT COUNSELING INFORMATION
- *
- Sections or subsections omitted from the full prescribing information are not listed.
-
BOXED WARNING
(What is this?)
WARNING: SUICIDAL THOUGHTS AND BEHAVIORS
- Antidepressants increased the risk of suicidal thoughts and behavior in pediatric and young adult patients in short-term studies.
Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [see Warnings and Precautions (5.1)].
-
1 INDICATIONS AND USAGE
DRIZALMA SPRINKLE is indicated for the treatment of:
- •
- Major Depressive Disorder in adults
- •
- Generalized Anxiety Disorder in adults and pediatric patients 7 years of age and older
- •
- Diabetic Peripheral Neuropathy in adults
- •
- Fibromyalgia in adults
- •
- Chronic Musculoskeletal Pain in adults
Additional pediatric use information is approved for Eli Lilly and Company, Inc.’s CYMBALTA (duloxetine delayed-release capsules). However, due to Eli Lilly and Company Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
-
2 DOSAGE AND ADMINISTRATION
2.1 Important Administration Instructions
Administer DRIZALMA SPRINKLE with or without food. Swallow DRIZALMA SPRINKLE whole (do not chew or crush the capsule). For patients unable to swallow an intact capsule, refer to the alternative administration instructions below.
Directions for use with applesauce
For patients with swallowing difficulty, DRIZALMA SPRINKLE can be opened and the contents sprinkled over applesauce. The contents of the capsules should be swallowed along with a small amount (tablespoonful) of applesauce. The drug/food mixture should be swallowed immediately and not stored for future use.
Nasogastric tube administration
Open and add contents of capsule to an all plastic catheter tip syringe and add 50 mL of water. Gently shake the syringe for approximately 10 seconds. Promptly deliver through a 12 French or larger nasogastric tube. Ensure no pellets are left in the syringe. Rinse with additional water (about 15 mL) if needed.
If a dose of DRIZALMA SPRINKLE is missed, take the missed dose as soon as it is remembered. If it is almost time for the next dose, skip the missed dose and take the next dose at the regular time. Do not take two doses of DRIZALMA SPRINKLE at the same time.
2.2 Dosage for Treatment of Major Depressive Disorder in Adults
The recommended starting dosage in adults with MDD is 40 mg per day (given as 20 mg twice daily) to 60 mg per day (given either once daily or as 30 mg twice daily). For some patients, it may be desirable to start at 30 mg once daily for 1 week, to allow patients to adjust to DRIZALMA SPRINKLE before increasing to 60 mg once daily. While a 120 mg per day dose was shown to be effective, there is no evidence that doses greater than 60 mg per day confer any additional benefits. Periodically reassess to determine the need for maintenance treatment and the appropriate dosage for such treatment [see Clinical Studies (14.1)].
2.3 Dosage for Treatment of Generalized Anxiety Disorder
Recommended Dosage in Adults Less than 65 Years of Age
For most adults less than 65 years of age with GAD, initiate DRIZALMA SPRINKLE 60 mg once daily. For some patients, it may be desirable to start at 30 mg once daily for 1 week, to allow patients to adjust to DRIZALMA SPRINKLE before increasing to 60 mg once daily. While a 120 mg once daily dosage was shown to be effective, there is no evidence that doses greater than 60 mg per day confer additional benefit. Nevertheless, if a decision is made to increase the dosage beyond 60 mg once daily, increase dosage in increments of 30 mg once daily. The safety of doses above 120 mg once daily has not been adequately evaluated. Periodically reassess to determine the continued need for maintenance treatment and the appropriate dosage for such treatment.
Recommended Dosage in Geriatric Patients
In geriatric patients with GAD, initiate DRIZALMA SPRINKLE at a dosage of 30 mg once daily for 2 weeks before considering an increase to the target dose of 60 mg per day. Thereafter, patients may benefit from doses above 60 mg once daily. If a decision is made to increase the dose beyond 60 mg once daily, increase dose in increments of 30 mg once daily. The maximum dose studied was 120 mg per day.
Recommended Dosage in Pediatric Patients 7 to 17 Years of Age
Initiate DRIZALMA SPRINKLE in pediatric patients 7 to 17 years of age with GAD at a dosage of 30 mg once daily for 2 weeks before considering an increase to 60 mg once daily. The recommended dosage range is 30 to 60 mg once daily. Some patients may benefit from dosages above 60 mg once daily. If a decision is made to increase the dose beyond 60 mg once daily, increase dosage in increments of 30 mg once daily. The maximum dose studied was 120 mg per day.
2.4 Dosage for Treatment of Diabetic Peripheral Neuropathic Pain in Adults
Administer DRIZALMA SPRINKLE 60 mg once daily in adults with diabetic peripheral neuropathic pain. There is no evidence that doses higher than 60 mg once daily confer additional significant benefit and the higher dosage is clearly less well tolerated. For patients for whom tolerability is a concern, a lower starting dose may be considered.
Since diabetes is frequently complicated by renal disease, consider a lower starting dosage and gradual increase in dosage for patients with renal impairment [see Dosage and Administration (2.8) and Use in Specific Populations (8.10)].
2.5 Dosage for Treatment of Fibromyalgia in Adults
Recommended Dosage in Adults
The recommended DRIZALMA SPRINKLE dosage is 60 mg once daily in adults with fibromyalgia. Begin treatment at 30 mg once daily for 1 week, to allow patients to adjust to DRIZALMA SPRINKLE before increasing to 60 mg once daily. Some patients may respond to the starting dosage. There is no evidence that dosages greater than 60 mg/day confer additional benefit, even in patients who do not respond to a 60 mg/day dosage, and higher dosages were associated with a higher rate of adverse reactions.
Additional pediatric use information is approved for Eli Lilly and Company, Inc.’s CYMBALTA (duloxetine delayed-release capsules). However, due to Eli Lilly and Company Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
2.6 Dosage for Treatment of Chronic Musculoskeletal Pain in Adults
The recommended DRIZALMA SPRINKLE dosage is 60 mg once daily in adults with chronic musculoskeletal pain. Begin treatment at 30 mg once daily for one week, to allow patients to adjust to DRIZALMA SPRINKLE before increasing to 60 mg once daily. There is no evidence that higher dosage confer additional benefit, even in patients who do not respond to a 60 mg once daily dosage, and higher dosages are associated with a higher rate of adverse reactions [see Clinical Studies (14.5)].
2.7 Dosage Recommendations for Concomitant Use with Potent CYP1A2 Inhibitors
Coadministration with potent CYP1A2 Inhibitors: Avoid concomitant use of DRIZALMA SPRINKLE with potent CYP1A2 inhibitors.
2.8 Dosage in Patients with Hepatic Impairment or Severe Renal Impairment
Avoid use in patients with chronic liver disease or cirrhosis [see Warnings and Precautions (5.14) and Use in Specific Populations (8.9)].
Avoid use in patients with severe renal impairment, GFR <30 mL/minute [see Warnings and Precautions (5.14) and Use in Specific Populations (8.10)].
2.9 Screen for Bipolar Disorder Prior to Starting DRIZALMA SPRINKLE
Prior to initiating treatment with DRIZALMA SPRINKLE or another antidepressant, screen patients for a personal or family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions (5.8)].
2.10 Discontinuing DRIZALMA SPRINKLE
Adverse reactions may occur upon discontinuation of DRIZALMA SPRINKLE [see Warnings and Precautions (5.7)]. Gradually reduce the dosage rather than stopping DRIZALMA SPRINKLE abruptly whenever possible [see Warnings and Precautions (5.7)].
2.11 Switching a Patient to or from a Monoamine Oxidase Inhibitor (MAOI) Intended to Treat Psychiatric Disorders
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with DRIZALMA SPRINKLE. Conversely, at least 5 days should be allowed after stopping DRIZALMA SPRINKLE before starting an MAOI intended to treat psychiatric disorders [see Contraindications (4).
2.12 Use of DRIZALMA SPRINKLE with Other MAOIs such as Linezolid or Methylene Blue
Do not start DRIZALMA SPRINKLE in a patient who is being treated with linezolid or intravenous methylene blue because there is an increased risk of serotonin syndrome. In a patient who requires more urgent treatment of a psychiatric condition, other interventions, including hospitalization, should be considered [see Contraindications (4)].
In some cases, a patient already receiving DRIZALMA SPRINKLE therapy may require urgent treatment with linezolid or intravenous methylene blue. If acceptable alternatives to linezolid or intravenous methylene blue treatment are not available and the potential benefits of linezolid or intravenous methylene blue treatment are judged to outweigh the risks of serotonin syndrome in a particular patient, DRIZALMA SPRINKLE should be stopped promptly, and linezolid or intravenous methylene blue can be administered. The patient should be monitored for symptoms of serotonin syndrome for 5 days or until 24 hours after the last dose of linezolid or intravenous methylene blue, whichever comes first. Therapy with DRIZALMA SPRINKLE may be resumed 24 hours after the last dose of linezolid or intravenous methylene blue [see Warnings and Precautions (5.4)].
The risk of administering methylene blue by non-intravenous routes (such as oral tablets or by local injection) or in intravenous doses much lower than 1 mg/kg with DRIZALMA SPRINKLE is unclear. The clinician should, nevertheless, be aware of the possibility of emergent symptoms of serotonin syndrome with such use [see Warnings and Precautions (5.4)].
-
3 DOSAGE FORMS AND STRENGTHS
20 mg: hard gelatin capsules with green cap imprinted with “RG53” and green body imprinted with “RG53” containing off-white to pale-yellow colored pellets. Each capsule contains 22.4 mg of duloxetine hydrochloride, USP equivalent to 20 mg duloxetine.
30 mg: hard gelatin capsules with blue cap imprinted with “RG54” and white body imprinted with “RG54” containing off-white to pale-yellow colored pellets. Each capsule contains 33.6 mg of duloxetine hydrochloride, USP equivalent to 30 mg duloxetine.
40 mg: hard gelatin capsules with white cap imprinted with “RL85” and white body imprinted with “RL 85” containing off-white to pale-yellow colored pellets. Each capsule contains 44.9 mg of duloxetine hydrochloride, USP equivalent to 40 mg duloxetine.
60 mg: hard gelatin capsules with blue cap imprinted with “RG55” and green body imprinted with “RG55” containing off-white to pale-yellow colored pellets. Each capsule contains 67.3 mg of duloxetine hydrochloride, USP equivalent to 60 mg duloxetine.
-
4 CONTRAINDICATIONS
The use of MAOIs intended to treat psychiatric disorders with DRIZALMA SPRINKLE, or within 5 days of stopping treatment with DRIZALMA SPRINKLE, are contraindicated because of an increased risk of serotonin syndrome. The use of DRIZALMA SPRINKLE within 14 days of stopping an MAOI intended to treat psychiatric disorders is contraindicated [see Dosage and Administration (2.10), Warnings and Precautions (5.4), Drug Interactions (7)].
Starting DRIZALMA SPRINKLE in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue is contraindicated because of an increased risk of serotonin syndrome [see Warnings and Precautions (5.4)].
-
5 WARNINGS AND PRECAUTIONS
5.1 Suicidal Thoughts and Behaviors in Adolescents and Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in the antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.Table 1: Risk Differences of the Number of Patients of Suicidal Thoughts and Behavior in the Pooled Placebo-Controlled Trials of Antidepressants in Pediatric and Adult Patients
Age Range Drug-Placebo Difference in Number of Patients of Suicidal Thoughts or Behaviors per 1000 Patients Treated Increases Compared to Placebo <18 14 additional patients 18 to 24 5 additional patients Decreases Compared to Placebo 25 to 64 1 fewer patients ≥65 6 fewer patients It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors. Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing DRIZALMA SPRINKLE, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
5.2 Hepatotoxicity
There have been reports of hepatic failure, sometimes fatal, in patients treated with duloxetine delayed-release capsules. These cases have presented as hepatitis with abdominal pain, hepatomegaly, and elevation of transaminase levels to more than twenty times the upper limit of normal (ULN) with or without jaundice, reflecting a mixed or hepatocellular pattern of liver injury. Discontinue DRIZALMA SPRINKLE in patients who develop jaundice or other evidence of clinically significant liver dysfunction and do not resume unless another cause can be established.
Cases of cholestatic jaundice with minimal elevation of transaminase levels have also been reported. Other post-marketing reports indicate that elevated transaminases, bilirubin, and alkaline phosphatase have occurred in patients with chronic liver disease or cirrhosis.
Duloxetine delayed-release capsules increased the risk of elevation of serum transaminase levels in development program clinical trials. Liver transaminase elevations resulted in the discontinuation of 0.3% (92/34,756) of duloxetine delayed-release capsules-treated patients. In most patients, the median time to detection of the transaminase elevation was about two months. In adult placebo-controlled trials in any indication, for patients with normal and abnormal baseline ALT values, elevation of ALT > 3 times the upper limit of normal occurred in 1.25% (144/11,496) of duloxetine delayed-release capsules-treated patients compared to 0.45% (39/8716) of placebo- treated patients. In adult placebo-controlled studies with duloxetine delayed-release capsules using a fixed dose design, there was evidence of a dose response relationship for ALT and AST elevation of >3 times the ULN and >5 times the ULN, respectively.
Because it is possible that duloxetine and alcohol may interact to cause liver injury or that duloxetine may aggravate pre-existing liver disease, DRIZALMA SPRINKLE should not be prescribed to patients with substantial alcohol use or evidence of chronic liver disease.
5.3 Orthostatic Hypotension, Falls and Syncope
Orthostatic hypotension, falls, and syncope have been reported in patients treated with the recommended duloxetine delayed-release capsules dosage. Syncope and orthostatic hypotension tend to occur within the first week of therapy but can occur at any time during DRIZALMA SPRINKLE treatment, particularly after dose increases. The risk of falling appears to be related to the degree of orthostatic decrease in blood pressure (BP) as well as other factors that may increase the underlying risk of falls.
In an analysis of patients from all placebo-controlled trials, patients treated with duloxetine delayed-release capsules reported a higher rate of falls compared to patients treated with placebo. Risk appears to be related to the presence of orthostatic decrease in BP. The risk of BP decreases may be greater in patients taking concomitant medications that induce orthostatic hypotension (such as anti-hypertensives) or are potent CYP1A2 inhibitors [see Warnings and Precautions (5.12) and Drug Interactions (7.1)] and in patients taking DRIZALMA SPRINKLE at doses above 60 mg daily. Consider dose reduction or discontinuation of DRIZALMA SPRINKLE in patients who experience symptomatic orthostatic hypotension, falls and/or syncope during DRIZALMA SPRINKLE therapy.
Risk of falling also appeared to be proportional to a patient’s underlying risk for falls and appeared to increase steadily with age. As geriatric patients tend to have a higher underlying risk for falls due to a higher prevalence of risk factors such as use of multiple medications, medical comorbidities and gait disturbances, the impact of increasing age by itself is unclear. Falls with serious consequences including fractures and hospitalizations have been reported with duloxetine delayed-release capsules use [see Adverse Reactions (6.1)].
5.4 Serotonin Syndrome
Serotonin-norepinephrine reuptake inhibitors (SNRIs) and selective-serotonin reuptake inhibitors (SSRIs), including DRIZALMA SPRINKLE, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, lithium, tramadol, meperidine, methadone, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin [see Contraindications (4), Drug Interactions (7)]. Serotonin syndrome can also occur when these drugs are used alone.
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of DRIZALMA SPRINKLE with MAOI antidepressant is contraindicated. In addition, do not initiate DRIZALMA SPRINKLE in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking DRIZALMA SPRINKLE, discontinue DRIZALMA SPRINKLE before initiating treatment with the MAOI [see Contraindications (4), Drug Interactions (7)].
Monitor all patients taking DRIZALMA SPRINKLE for the emergence of serotonin syndrome. Discontinue treatment with DRIZALMA SPRINKLE capsules immediately if the above symptoms occur and initiate supportive symptomatic treatment. If concomitant use of DRIZALMA SPRINKLE with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
5.5 Increased Risk of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including DRIZALMA SPRINKLE, may increase the risk of bleeding events. Concomitant use of aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), warfarin, and other anti-coagulants may add to this risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. A post-marketing study showed a higher incidence of postpartum hemorrhage in mothers taking duloxetine. Other bleeding events related to SSRIs and SNRIs use have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the increased risk of bleeding associated with the concomitant use of DRIZALMA SPRINKLE and NSAIDs, aspirin, or other drugs that affect coagulation [see Drug Interactions (7.1)].
5.6 Severe Skin Reactions
Severe skin reactions, including erythema multiforme and Stevens-Johnson Syndrome (SJS), can occur with DRIZALMA SPRINKLE. The reporting rate of SJS associated with duloxetine use exceeds the general population background incidence rate for this serious skin reaction (1 to 2 cases per million person years). The reporting rate is generally accepted to be an underestimate due to underreporting.
DRIZALMA SPRINKLE should be discontinued at the first appearance of blisters, peeling rash, mucosal erosions, or any other sign of hypersensitivity if no other etiology can be identified.
5.7 Discontinuation Syndrome
Discontinuation symptoms have been systematically evaluated in patients taking duloxetine. Following abrupt or tapered discontinuation in adult placebo-controlled clinical trials, the following symptoms occurred at 1% or greater and at a significantly higher rate in duloxetine-treated patients compared to those discontinuing from placebo: dizziness, headache, nausea, diarrhea, paresthesia, irritability, vomiting, insomnia, anxiety, hyperhidrosis, and fatigue.
Adverse reactions after discontinuation of serotonergic antidepressants, particularly after abrupt discontinuation, include: nausea, sweating, dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias, such as electric shock sensations), tremor, anxiety, confusion, headache, lethargy, emotional lability, insomnia, hypomania, tinnitus, and seizures.
Monitor patients for these symptoms when discontinuing treatment with DRIZALMA SPRINKLE [see Dosage and Administration (2.10)]. A gradual reduction in dosage rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the healthcare provider may continue decreasing the dose but at a more gradual rate [see Dosage and Administration (2.9)].
5.8 Activation of Mania/Hypomania
In patients with bipolar disorder, treating a depressive episode with DRIZALMA SPRINKLE or another antidepressant may precipitate a mixed/manic episode. In controlled clinical trials in adult patients with major depressive disorder, patients with bipolar disorder were generally excluded; however, symptoms of mania or hypomania were reported in 0.1% of patients treated with duloxetine delayed-release capsules. No activation of mania or hypomania was reported in DPNP, GAD, fibromyalgia, or chronic musculoskeletal pain placebo-controlled trials.
Prior to initiating treatment with DRIZALMA SPRINKLE, screen patients for any personal or family history of bipolar disorder, mania, or hypomania.
5.9 Angle-Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs, including DRIZALMA SPRINKLE, may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy. Avoid use of antidepressants, including DRIZALMA SPRINKLE, in patients with anatomically narrow angles.
5.10 Seizures
Duloxetine has not been systematically evaluated in patients with a seizure disorder, and such patients were excluded from clinical studies. In adult placebo-controlled clinical trials, seizures/convulsions occurred in 0.02% (3/12,722) of patients treated with duloxetine delayed-release capsules and 0.01% (1/9513) of patients treated with placebo. DRIZALMA SPRINKLE should be prescribed with care in patients with a history of a seizure disorder.
5.11 Increases in Blood Pressure
In adult placebo-controlled clinical trials across the approved adult populations from baseline to endpoint, duloxetine treatment was associated with mean increases of 0.5 mm Hg in systolic blood pressure and 0.8 mm Hg in diastolic blood pressure compared to mean decreases of 0.6 mm Hg systolic and 0.3 mm Hg diastolic in placebo-treated patients. There was no significant difference in the frequency of sustained (3 consecutive visits) elevated blood pressure.
Patients receiving DRIZALMA SPRINKLE should have regular monitoring of blood pressure since increases in blood pressure were observed in clinical studies [see Adverse Reactions (6.1)]. Pre-existing hypertension should be controlled before initiating treatment with DRIZALMA SPRINKLE. Caution should be exercised in treating patients with pre-existing hypertension, cardiovascular, or cerebrovascular conditions that might be compromised by increases in blood pressure.
Sustained blood pressure increases could have adverse consequences. For patients who experience a sustained increase in blood pressure while receiving DRIZALMA SPRINKLE, either dose reduction or discontinuation should be considered [see Adverse Reactions (6.1), Drug Interactions (7.1)]].
5.12 Clinically Important Drug Interactions
Both CYP1A2 and CYP2D6 are responsible for duloxetine metabolism.
Potential for Other Drugs to Affect DRIZALMA SPRINKLE
CYP1A2 Inhibitors – Avoid concomitant use of DRIZALMA SPRINKLE with potent CYP1A2 inhibitors [see Drug Interactions (7.1)].
CYP2D6 Inhibitors - Concomitant use of DRIZALMA SPRINKLE with potent inhibitors of CYP2D6 would be expected to, and does, result in higher concentrations (on average of 60%) of duloxetine [see Drug Interactions (7.1)].
Potential for DRIZALMA SPRINKLE to Affect Other Drugs
Drugs Metabolized by CYP2D6 - Co-administration of DRIZALMA SPRINKLE with drugs that are extensively metabolized by CYP2D6 and that have a narrow therapeutic index, including certain antidepressants (tricyclic antidepressants [TCAs], such as nortriptyline, amitriptyline, and imipramine), phenothiazines and Type 1C antiarrhythmics (e.g., propafenone, flecainide), should be approached with caution. Plasma TCA concentrations may need to be monitored and the dose of the TCA may need to be reduced if a TCA is co-administered with DRIZALMA SPRINKLE. Because of the risk of serious ventricular arrhythmias and sudden death potentially associated with elevated plasma levels of thioridazine, DRIZALMA SPRINKLE and thioridazine should not be co-administered [see Drug Interactions (7.1)].
Other Clinically Important Drug Interactions
Alcohol - Use of DRIZALMA SPRINKLE concomitantly with heavy alcohol intake may be associated with severe liver injury. For this reason, DRIZALMA SPRINKLE should not be prescribed for patients with substantial alcohol use [see Warnings and Precautions (5.2)].
When duloxetine and ethanol were administered several hours apart so that peak concentrations of each would coincide, duloxetine did not increase the impairment of mental and motor skills caused by alcohol.
In the duloxetine clinical trials database, three duloxetine-treated patients had liver injury as manifested by ALT and total bilirubin elevations, with evidence of obstruction. Substantial intercurrent ethanol use was present in each of these cases, and this may have contributed to the abnormalities seen [see Warnings and Precautions (5.2), Drug Interactions (7.1)].
CNS Acting Drugs - Given the primary CNS effects of duloxetine, DRIZALMA SPRINKLE should be used with caution when it is taken in combination with or substituted for other centrally acting drugs, including those with a similar mechanism of action [see Drug Interactions (7.1)].
5.13 Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs and SNRIs, including DRIZALMA SPRINKLE. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH). Cases with serum sodium lower than 110 mmol/L have been reported. Geriatric patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted may be at greater risk [see Use in Specific Populations (8.5)]. Discontinuation of DRIZALMA SPRINKLE should be considered in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have been included hallucination, syncope, seizure, coma, respiratory arrest, and death.
5.14 Use in Patients with Concomitant Illness
Clinical experience with duloxetine delayed-release capsules in patients with concomitant systemic illnesses is limited. There is no information on the effect that alterations in gastric motility may have on the stability of DRIZALMA SPRINKLE enteric coating. In extremely acidic conditions, duloxetine, unprotected by the enteric coating, may undergo hydrolysis to form naphthol. Caution is advised in using DRIZALMA SPRINKLE in patients with conditions that may slow gastric emptying (e.g., some diabetics).
DRIZALMA SPRINKLE has not been systematically evaluated in patients with a recent history of myocardial infarction or unstable coronary artery disease. Patients with these diagnoses were generally excluded from clinical studies during the product’s premarketing testing.
Hepatic Impairment
Avoid use in patients with chronic liver disease or cirrhosis [see Dosage and Administration (2.7), Warnings and Precautions (5.2), and Use in Specific Populations (8.9)].
Severe Renal Impairment
Avoid use in patients with severe renal impairment, GFR < 30 mL/minute. Increased plasma concentration of duloxetine, and especially of its metabolites, occur in patients with end-stage renal disease (requiring dialysis) [see Dosage and Administration (2.7) and Use in Specific Populations (8.10)].
Glycemic Control in Patients with Diabetes
As observed in DPNP trials, duloxetine delayed-release capsules treatment worsened glycemic control in some patients with diabetes. In three clinical trials of duloxetine delayed-release capsules for the management of neuropathic pain associated with diabetic peripheral neuropathy [see clinical studies (14.3)], the mean duration of diabetes was approximately 12 years, the mean baseline fasting blood glucose was 176 mg/dL, and the mean baseline hemoglobin A1c (HbA1c) was 7.8%. In the 12 week acute treatment phase of these studies, duloxetine delayed-release capsules was associated with a small increase in mean fasting blood glucose as compared to placebo. In the extension phase of these studies, which lasted up to 52 weeks, mean fasting blood glucose increased by 12 mg/dL in the duloxetine delayed-release capsules group and decreased by 11.5 mg/dL in the routine care group. HbA1c increased by 0.5% in the duloxetine delayed-release group and by 0.2% in the routine care group.
5.15 Urinary Hesitation and Retention
DRIZALMA SPRINKLE is in a class of drugs known to affect urethral resistance. If symptoms of urinary hesitation develop during treatment with DRIZALMA SPRINKLE, consideration should be given to the possibility that they might be drug-related.
In post marketing experience, cases of urinary retention have been observed. In some instances of urinary retention associated with duloxetine delayed-release capsules use, hospitalization and/or catheterization has been needed.
5.16 Sexual Dysfunction
Use of SNRIs, including DRIZALMA SPRINKLE, may cause symptoms of sexual dysfunction [see Adverse Reactions (6.1)]. In male patients, SNRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SNRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of DRIZALMA SPRINKLE and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
-
6 ADVERSE REACTIONS
The following adverse reactions are discussed in more detail in other sections of the labeling:
- Suicidal Thoughts and Behaviors in Adolescents and Young Adults [see Boxed Warning and Warnings and Precautions (5.1)]
- Hepatotoxicity [see Warnings and Precautions (5.2)]
- Orthostatic Hypotension, Falls and Syncope [see Warnings and Precautions (5.3)]
- Serotonin Syndrome [see Warnings and Precautions (5.4)]
- Increased Risk of Bleeding [see Warnings and Precautions (5.5)]
- Severe Skin Reactions [see Warnings and Precautions (5.6)]
- Discontinuation Syndrome [see Warnings and Precautions (5.7)]
- Activation of Mania/Hypomania [see Warnings and Precautions (5.8)]
- Angle-Closure Glaucoma [see Warnings and Precautions (5.9)]
- Seizures [see Warnings and Precautions (5.10)]
- Increases in Blood Pressure [see Warnings and Precautions (5.11)]
- Clinically Important Drug Interactions [see Warnings and Precautions (5.12)]
- Hyponatremia [see Warnings and Precautions (5.13)]
- Urinary Hesitation and Retention [see Warnings and Precautions (5.15)]
- Sexual Dysfunction [see Warnings and Precautions (5.16)]
6.1 Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The stated frequencies of adverse reactions represent the proportion of patients who experienced, at least once, a treatment-emergent adverse reaction of the type listed.
Adverse Reactions in Adults
The data described below reflect exposure to duloxetine delayed-release capsules in placebo-controlled trials for MDD (N = 3779), GAD (N = 1018), OA (N = 503), CLBP (N = 600), and DPNP (N = 906), and FM (N = 1294). The population studied was 17 to 89 years of age; 65.7%, 60.8%, 60.6%, 42.9%, and 94.4% female; and 81.8%, 72.6%, 85.3%, 74.0%, and 85.7% Caucasian for MDD, GAD, OA and CLBP, DPNP, and FM, respectively. Most patients received doses of a total of 60 to 120 mg per day [see Clinical Studies (14)]. The data below do not include results of the trial examining the efficacy of duloxetine delayed-release in patients ≥ 65 years old for the treatment of generalized anxiety disorder; however, the adverse reactions observed in this geriatric sample were generally similar to adverse reactions in the overall adult population.
Adverse Reactions Reported as Reasons for Discontinuation of Treatment in Adult Placebo-Controlled Trials
Major Depressive Disorder:
Approximately 8.4% (319/3779) of the duloxetine delayed-release capsules-treated patients in placebo-controlled adult trials for MDD discontinued treatment due to an adverse reaction, compared with 4.6% (117/2536) of placebo-treated patients. Nausea (duloxetine delayed-release capsules 1.1%, placebo 0.4%) was the only adverse reaction reported as a reason for discontinuation and considered to be drug-related (i.e., discontinuation occurring in at least 1% of the duloxetine delayed-release capsules-treated patients and at a rate of at least twice that of placebo).
Generalized Anxiety Disorder:
Approximately 13.7% (139/1018) of the duloxetine delayed-release capsules-treated patients in placebo-controlled adult trials for GAD discontinued treatment due to an adverse reaction, compared with 5.0% (38/767) of placebo-treated patients. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine delayed-release capsules 3.3%, placebo 0.4%), and dizziness (duloxetine delayed-release capsules 1.3%, placebo 0.4%).
Diabetic Peripheral Neuropathic Pain:
Approximately 12.9% (117/906) of the duloxetine delayed-release capsules-treated patients in placebo-controlled adult trials for DPNP discontinued treatment due to an adverse reaction, compared with 5.1% (23/448) for placebo-treated patients. Common adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine delayed-release capsules 3.5%, placebo 0.7%), dizziness (duloxetine delayed-release capsules 1.2%, placebo 0.4%), and somnolence (duloxetine delayed-release capsules 1.1%, placebo 0.0%).
Fibromyalgia:
Approximately 17.5% (227/1294) of the duloxetine delayed-release capsules-treated patients in 3-to 6-month placebo-controlled adult trials for FM discontinued treatment due to an adverse reaction, compared with 10.1% (96/955) for placebo-treated patients. Adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine delayed-release capsules 2.0%, placebo 0.5%), headache (duloxetine delayed-release capsules 1.2%, placebo 0.3%), somnolence (duloxetine delayed-release capsules 1.1%, placebo 0%), and fatigue (duloxetine delayed-release capsules 1.1%, placebo 0.1%).
Chronic Pain due to Osteoarthritis:
Approximately 15.7% (79/503) of the duloxetine delayed-release capsules-treated patients in 13 week, placebo-controlled adult trials for chronic pain due to OA discontinued treatment due to an adverse reaction, compared with 7.3% (37/508) for placebo-treated patients. Adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine delayed-release capsules 2.2%, placebo 1.0%).
Chronic Low Back Pain:
Approximately 16.5% (99/600) of the duloxetine delayed-release capsules-treated patients in 13 week, placebo-controlled adult trials for CLBP discontinued treatment due to an adverse reaction, compared with 6.3% (28/441) for placebo-treated patients. Adverse reactions reported as a reason for discontinuation and considered to be drug-related (as defined above) included nausea (duloxetine delayed-release capsules 3.0%, placebo 0.7%), and somnolence (duloxetine delayed-release capsules 1.0%, placebo 0.0%).
Most Common Adverse Reactions (Adults)Diabetic Peripheral Neuropathic Pain: nausea, somnolence, decreased appetite, constipation, hyperhidrosis, and dry mouth.
Fibromyalgia: nausea, dry mouth, constipation, somnolence, decreased appetite, hyperhidrosis, and agitation.
Chronic Pain due to Osteoarthritis: nausea, fatigue, constipation, dry mouth, insomnia, somnolence, and dizziness.
Chronic Low Back Pain: nausea, dry mouth, insomnia, somnolence, constipation, dizziness, and fatigue.
Adverse Reactions Occurring at an Incidence of 5% or More Among Duloxetine Delayed-Release Capsules Treated Patients in Adult Placebo-Controlled TrialsThe most commonly observed adverse reactions in duloxetine delayed-release capsule-treated patients (incidence of at least 5% and at least twice the incidence in placebo patients) were nausea, dry mouth, somnolence, constipation, decreased appetite, and hyperhidrosis. Table 2 displays the incidence of adverse reactions in placebo-controlled trials for approved indications that occurred in 5% or more of patients treated with duloxetine delayed-release capsules and with an incidence greater than placebo-treated patients.
Table 2: Adverse Reactions: Incidence of 5% or More and Greater than Placebo in Placebo-Controlled Trials of Approved Adult Populationsa
Adverse Reaction
Percentage of Patients Reporting Reaction
Duloxetine delayed-release capsules
(N = 8100)
Placebo
(N = 5655)
Nauseac
23
8
Headache
14
12
Dry mouth
13
5
Somnolencee
10
3
Fatigueb,c
9
5
Insomniad
9
5
Constipationc
9
4
Dizzinessc
9
5
Diarrhea
9
6
Decreased appetitec
7
2
Hyperhidrosisc
6
1
Abdominal painf
5
4
a Includes adults with MDD, GAD, DPNP, FM, and chronic musculoskeletal pain. The inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
b Also includes asthenia.
c Events for which there was a significant dose-dependent relationship in fixed-dose studies, excluding three MDD studies which did not have a placebo lead-in period or dose titration.
d Also includes initial insomnia, middle insomnia, and early morning awakening.
e Also includes hypersomnia and sedation.
f Also includes abdominal discomfort, abdominal pain lower, abdominal pain upper, abdominal tenderness, and gastrointestinal pain.
Adverse Reactions in Pooled MDD and GAD Trials in AdultsTable 3 displays the incidence of adverse reactions in MDD and GAD placebo-controlled trials for approved indications that occurred in 2% or more of patients treated with duloxetine delayed-release capsules and with an incidence greater than placebo-treated patients.
Table 3: Adverse Reactions: Incidence of 2% or More and Greater than Placebo in MDD and GAD Placebo-Controlled Trials in Adultsa,b
System Organ Class / Adverse Reaction
Percentage of Patients Reporting Reaction
Duloxetine delayed-release capsules
(N = 4797)
Placebo
(N = 3303)
Cardiac Disorders
Palpitations
2
1
Eye Disorders
Vision blurred
3
1
Gastrointestinal Disorders
Nauseac
23
8
Dry mouth
14
6
Constipationc
9
4
Diarrhea
9
6
Abdominal paind
5
4
Vomiting
4
2
General Disorders and Administration Site
Conditions
Fatiguee
9
5
Metabolism and Nutrition Disorders
Decreased appetitec
6
2
Nervous System Disorders
Headache
14
14
Dizzinessc
9
5
Somnolencef
9
3
Tremor
3
1
Psychiatric Disorders
Insomniag
9
5
Agitationh
4
2
Anxiety
3
2
Reproductive System and Breast Disorders
Erectile dysfunction
4
1
Ejaculation delayedc
2
1
Libido decreasedi
3
1
Orgasm abnormalj
2
<1
Respiratory, Thoracic, and Mediastinal Disorders
Yawning
2
<1
Skin and Subcutaneous Tissue Disorders
Hyperhidrosis
6
2
a The inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
b For GAD, there were no adverse reactions that were significantly different between treatments in adults ≥65 years that were also not significant in the adults <65 years.
c Events for which there was a significant dose-dependent relationship in fixed-dose studies, excluding three MDD studies which did not have a placebo lead-in period or dose titration.
d Includes abdominal pain upper, abdominal pain lower, abdominal tenderness, abdominal discomfort, and gastrointestinal pain
e Includes asthenia
f Includes hypersomnia and sedation
g Includes initial insomnia, middle insomnia, and early morning awakening
h Includes feeling jittery, nervousness, restlessness, tension and psychomotor hyperactivity
i Includes loss of libido
j Includes anorgasmia
Adverse Reactions in the DPNP, FM, OA, and CLBP Adult Trials:
Table 4 displays the incidence of adverse reactions that occurred in 2% or more of duloxetine delayed-release capsules-treated patients (determined prior to rounding) in the premarketing acute phase of DPNP, FM, OA, and CLBP placebo-controlled adult trials and with an incidence greater than placebo-treated patients.
Table 4: Adverse Reactions: Incidence of 2% or More and Greater than Placebo in DPNP, FM, OA, and CLBP Placebo-Controlled Trials in Adultsa
System Organ Class / Adverse Reaction
Percentage of Patients Reporting Reaction
Duloxetine delayed-release capsules
(N = 3303)
Placebo
(N = 2352)
Gastrointestinal Disorders
Nausea
Dry Mouthb
Constipationb
Diarrhea
Abdominal Painc
Vomiting
Dyspepsia
23
11
10
9
5
3
2
7
3
3
5
4
2
1
General Disorders and Administration Site Conditions
Fatigued
11
5
Infections and Infestations
Nasopharyngitis
Upper Respiratory Tract Infection
Influenza
4
3
2
4
3
2
Metabolism and Nutrition Disorders
Decreased Appetiteb
8
1
Musculoskeletal and Connective Tissue
Musculoskeletal Paine
Muscle Spasms
3
2
3
2
Nervous System Disorders
Headache Somnolenceb,f Dizziness Paraesthesiag Tremorb
13
11
9
2
2
8
3
5
2
<1
Psychiatric Disorders
Insomniab,h
Agitationi
10
3
5
1
Reproductive System and Breast Disorders
Erectile Dysfunctionb
Ejaculation Disorderj
4
2
<1
<1
Respiratory, Thoracic, and Mediastinal Disorders
Cough
2
2
Skin and Subcutaneous Tissue Disorders
Hyperhidrosis
6
1
Vascular Disorders
Flushingk
Blood pressure increasedl
3
2
1
1
a The inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
b Incidence of 120 mg/day is significantly greater than the incidence for 60 mg/day.
c Includes abdominal discomfort, abdominal pain lower, abdominal pain upper, abdominal tenderness and gastrointestinal pain
d Includes asthenia
e Includes myalgia and neck pain
f Includes hypersomnia and sedation
g Includes hypoaesthesia, hypoaesthesia facial, genital hypoaesthesia and paraesthesia oral
h Includes initial insomnia, middle insomnia, and early morning awakening.
i Includes feeling jittery, nervousness, restlessness, tension and psychomotor hyperactivity
j Includes ejaculation failure
k Includes hot flush
l Includes blood pressure diastolic increased, blood pressure systolic increased, diastolic hypertension, essential hypertension, hypertension, hypertensive crisis, labile hypertension, orthostatic hypertension, secondary hypertension, and systolic hypertension
Effects on Male and Female Sexual Function in Adults with MDDChanges in sexual desire, sexual performance and sexual satisfaction often occur as manifestations of psychiatric disorders or diabetes, but they may also be a consequence of pharmacologic treatment. Because adverse sexual reactions are presumed to be voluntarily underreported, the Arizona Sexual Experience Scale (ASEX), a validated measure designed to identify sexual side effects, was used prospectively in 4 MDD placebo-controlled adult trials [see Clinical Studies (14.1)]. The ASEX scale includes five questions that pertain to the following aspects of sexual function: 1) sex drive, 2) ease of arousal, 3) ability to achieve erection (men) or lubrication (women), 4) ease of reaching orgasm, and 5) orgasm satisfaction. Positive numbers signify a worsening of sexual function from baseline. Negative numbers signify an improvement from baseline level of sexual dysfunction from baseline, which is commonly seen in depressed patients.
In these trials, male patients treated with duloxetine delayed-release capsules experienced significantly more sexual dysfunction, as measured by the total score on the ASEX and the ability to reach orgasm, than did placebo-treated male patients (see Table 5). Female patients treated with duloxetine delayed-release capsules did not experience more sexual dysfunction than on placebo-treated female patients as measured by ASEX total score. Healthcare providers should routinely inquire about possible sexual adverse reactions in patients treated with duloxetine delayed-release capsules.
Table 5: Mean Change in ASEX Scores by Gender in MDD Placebo-Controlled Adult Trials
Male Patientsa
Female Patientsa
Duloxetine delayed-release capsules
(n = 175)
Placebo
(n = 83)
Duloxetine delayed-release capsules
(n = 241)
Placebo
(n = 126)
ASEX Total (Items 1-5)
Item 1 - Sex drive
Item 2 - Arousal
Item 3 - Ability to achieve erection (men); Lubrication (women)
Item 4 - Ease of reaching orgasm
Item 5 - Orgasm satisfaction
0.56b
-0.07
0.01
0.03
0.40c
0.09
-1.07
-0.12
-0.26
-0.25
-0.24
-0.13
-1.15
-0.32
-0.21
-0.17
-0.09
-0.11
-1.07
-0.24
-0.18
-0.18
-0.13
-0.17
a n = Number of patients with non-missing change score for ASEX total
b p = 0.013 versus placebo
c p<0.001 versus placebo
Vital Sign Changes in AdultsIn placebo-controlled clinical trials across approved adult populations for change from baseline to endpoint, duloxetine delayed-release capsules-treated patients had mean increases of 0.23 mm Hg in systolic blood pressure (SBP) and 0.73 mm Hg in diastolic blood pressure (DBP) compared to mean decreases of 1.09 mm Hg SBP and 0.55 mm Hg in DBP placebo-treated patients. There was no significant difference in the frequency of sustained (3 consecutive visits) elevated blood pressure [see Warnings and Precautions (5.3, 5.11)].
Duloxetine delayed-release capsules treatment, for up to 26 weeks in placebo-controlled trials across approved adult populations, typically caused a small increase in heart rate for change from baseline to endpoint compared to placebo of up to 1.37 beats per minute (increase of 1.20 beats per minute in duloxetine delayed-release capsules-treated patients, decrease of 0.17 beats per minute in placebo-treated patients).
Laboratory Changes in AdultsDuloxetine delayed-release capsules treatment in placebo-controlled clinical trials across approved adult populations, was associated with small mean increases from baseline to endpoint in ALT, AST, CPK, and alkaline phosphatase; infrequent, modest, transient, abnormal values were observed for these analytes in duloxetine delayed-release capsules-treated patients when compared with placebo-treated patients [see Warnings and Precautions (5.2)]. High bicarbonate, cholesterol, and abnormal (high or low) potassium, were observed more frequently in duloxetine delayed-release capsules treated patients compared to placebo-treated patients.
Other Adverse Reactions Observed During the Premarketing and Postmarketing Clinical Trial Evaluation of Duloxetine Delayed-Release Capsules in AdultsFollowing is a list of adverse reactions reported by patients treated with duloxetine delayed-release capsules in clinical trials. In clinical trials of all approved adult populations, 34,756 patients were treated with duloxetine delayed-release capsules. Of these, 26.9% (9337) took duloxetine delayed-release capsules for at least 6 months, and 12% (4317) for at least one year. The following listing is not intended to include reactions (1) already listed in previous tables or elsewhere in labeling, (2) for which a drug cause was remote, (3) which were so general as to be uninformative, (4) which were not considered to have significant clinical implications, or (5) which occurred at a rate equal to or less than placebo.
Reactions are categorized by body system according to the following definitions: frequent adverse reactions are those occurring in at least 1/100 patients; infrequent adverse reactions are those occurring in 1/100 to 1/1000 patients; rare reactions are those occurring in fewer than 1/1000 patients.
Cardiac Disorders- Frequent:palpitations; Infrequent: myocardial infarction, tachycardia, and Takotsubo cardiomyopathy.
Ear and Labyrinth Disorders- Frequent:vertigo; Infrequent: ear pain and tinnitus.
Endocrine Disorders- Infrequent:hypothyroidism.
Eye Disorders- Frequent:vision blurred; Infrequent: diplopia, dry eye, and visual impairment.
Gastrointestinal Disorders- Frequent: flatulence; Infrequent: dysphagia, eructation, gastritis, gastrointestinal hemorrhage, halitosis, and stomatitis; Rare: gastric ulcer.
General Disorders and Administration Site Conditions- Frequent: chills/rigors; Infrequent: falls, feeling abnormal, feeling hot and/or cold, malaise, and thirst; Rare: gait disturbance.
Infections and Infestations- Infrequent: gastroenteritis and laryngitis.
Investigations - Frequent: weight increased, weight decreased; Infrequent: blood cholesterol increased.
Metabolism and Nutrition Disorders- Infrequent: dehydration and hyperlipidemia; Rare: dyslipidemia.
Musculoskeletal and Connective Tissue Disorders- Frequent: musculoskeletal pain; Infrequent: muscle tightness and muscle twitching.
Nervous System Disorders- Frequent: dysgeusia, lethargy, and paraesthesia/hypoesthesia; Infrequent:
disturbance in attention, dyskinesia, myoclonus, and poor quality sleep; Rare: dysarthria.
Psychiatric Disorders- Frequent: abnormal dreams and sleep disorder; Infrequent: apathy, bruxism, disorientation/confusional state, irritability, mood swings, and suicide attempt; Rare: completed suicide.
Renal and Urinary Disorders- Frequent: urinary frequency; Infrequent: dysuria, micturition urgency, nocturia, polyuria, and urine odor abnormal.
Reproductive System and Breast Disorders- Frequent: anorgasmia/orgasm abnormal; Infrequent:
menopausal symptoms, sexual dysfunction, and testicular pain; Rare: menstrual disorder.
Respiratory, Thoracic and Mediastinal Disorders- Frequent: yawning, oropharyngeal pain; Infrequent: throat tightness.
Skin and Subcutaneous Tissue Disorders- Frequent: pruritus; Infrequent: cold sweat, dermatitis contact, erythema, increased tendency to bruise, night sweats, and photosensitivity reaction; Rare: ecchymosis.
Vascular Disorders- Frequent: hot flush; Infrequent: flushing, orthostatic hypotension, and peripheral coldness.
Adverse Reactions Observed in Placebo-Controlled Clinical Trials in Pediatric PatientsThe data described below reflect exposure to duloxetine delayed-release capsules in pediatric, 10 week, placebo-controlled trials for MDD (N = 341) and GAD (N = 135). The population studied (N = 476) was 7 to 17 years of age with 42.4% children age 7 to 11 years of age, 50.6% female, and 68.6% white. Patients received 30 to 120 mg per day during placebo-controlled acute treatment studies. Additional data come from the overall total of 822 pediatric patients (age 7 to 17 years of age) with 41.7% children age 7 to 11 years of age and 51.8% female exposed to duloxetine delayed-release capsules in MDD and GAD clinical trials up to 36 weeks in length, in which most patients received 30 to 120 mg per day. The safety and effectiveness of DRIZALMA SPRINKLE have not been established in pediatric patients with major depressive disorder (MDD), diabetic peripheral neuropathic pain, or chronic musculoskeletal pain.
Pediatric Clinical Trial Database
The adverse drug reaction profile observed in pediatric clinical trials in pediatric patients aged 7 to 17 years old was consistent with the adverse drug reaction profile observed in adult clinical trials. The specific adverse drug reactions observed in adult patients can be expected to be observed in pediatric patients [see Adverse Reactions (6.1)]. The most common (≥5% and twice placebo) adverse reactions observed in pediatric clinical trials include: nausea, diarrhea, decreased weight, and dizziness.
Adverse Reactions in Pediatric Patients Aged 7 to 17 Years Old with MDD and GAD
Table 6 provides the incidence of adverse reactions in MDD and GAD pediatric placebo- controlled trials that occurred in greater than 2% of patients treated with duloxetine delayed-release capsules and with an incidence greater than placebo. DRIZALMA SPRINKLE is not approved for the treatment of MDD in pediatric patients [see Use in Specific Populations(8.4)]
Table 6: Adverse Reactions: Incidence of 2% or More and Greater than Placebo in three 10 week Pediatric Placebo-Controlled Trialsin MDDa and GADb
System Organ Class/Adverse Reaction
Percentage of Pediatric Patients Reporting Reaction
Duloxetine delayed-release capsules
(N = 476)
Placebo (N = 362)
Gastrointestinal Disorders
Nausea
Abdominal Painc
Vomiting
Diarrhea
Dry Mouth
18
13
9
6
2
8
10
4
3
1
General Disorders and Administration Site
Conditions
Fatigued
7
5
Investigations
Decreased Weighte
14
6
Metabolism and Nutrition Disorders
Decreased Appetite
10
5
Nervous System Disorders
Headache
Somnolencef
Dizziness
18
11
8
13
6
4
Psychiatric Disorders
Insomniag
7
4
Respiratory, Thoracic, and Mediastinal
Disorders
Oropharyngeal Pain
Cough
4
3
2
1
a DRIZALMA SPRINKLE is not approved for the treatment of pediatric MDD [see Use in Specific Populations(8.4)].
bThe inclusion of an event in the table is determined based on the percentages before rounding; however, the percentages displayed in the table are rounded to the nearest integer.
c Also includes abdominal pain upper, abdominal pain lower, abdominal tenderness, abdominal discomfort, and gastrointestinal pain.
d Also includes asthenia.
e Frequency based on weight measurement meeting potentially clinically significant threshold of ≥3.5% weight loss
(N = 467 duloxetine delayed-release capsules; N = 354 Placebo).
f Also includes hypersomnia and sedation.
g Also includes initial insomnia, insomnia, middle insomnia, and terminal insomnia.
Other adverse reactions that occurred at an incidence of less than 2% and were reported by more duloxetine delayed-release capsules treated patients than placebo treated patients in pediatric MDD and GAD clinical trials included abnormal dreams (including nightmare), anxiety, flushing (including hot flush), hyperhidrosis, palpitations, pulse increased, and tremor (DRIZALMA SPRINKLE is not approved for the treatment of pediatric MDD).
The most commonly reported symptoms following discontinuation of duloxetine delayed-release capsules in pediatric clinical trials have included headache, dizziness, insomnia, and abdominal pain [see Warnings and Precautions (5.7)].
Growth (Height and Weight) in Pediatric Patients 7 to 17 Years Old with GAD and MDD
Decreased appetite and weight loss have been observed in association with the use of SSRIs and SNRIs. Pediatric patients treated with duloxetine delayed-release capsules in clinical trials experienced a 0.1kg mean decrease in weight at 10 weeks, compared with a mean weight gain of approximately 0.9 kg in placebo-treated patients. The proportion of patients who experienced a clinically significant decrease in weight (≥3.5%) was greater in the duloxetine delayed-release capsules group than in the placebo group (16% and 6%, respectively). Subsequently, over the 4- to 6-month uncontrolled extension periods, duloxetine delayed-release capsules-treated patients on average trended toward recovery to their expected baseline weight percentile based on population data from age- and sex-matched peers.
In studies up to 9 months, duloxetine delayed-release capsules-treated pediatric patients experienced an increase in height of 1.7 cm on average (2.2 cm increase in patients [7 to 11 years of age] and 1.3 cm increase in patients [12 to 17 years of age]). While height increase was observed during these studies, a mean decrease of 1% in height percentile was observed (decrease of 2% in patients [7 to 11 years of age] and increase of 0.3% in patients [12 to 17 years of age]). Weight and height should be monitored regularly in pediatric patients treated with DRIZALMA SPRINKLE.
Additional pediatric use information is approved for Eli Lilly and Company, Inc.’s CYMBALTA (duloxetine delayed-release capsules). However, due to Eli Lilly and Company Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
6.2 Postmarketing Experience
The following adverse reactions have been identified during post approval use of duloxetine delayed-release capsules. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Adverse reactions reported since market introduction that were temporally related to duloxetine delayed-release capsules therapy and not mentioned elsewhere in labeling include: acute pancreatitis, anaphylactic reaction, aggression and anger (particularly early in treatment or after treatment discontinuation), angioneurotic edema, angle-closure glaucoma, colitis (microscopic or unspecified), cutaneous vasculitis (sometimes associated with systemic involvement), extrapyramidal disorder, galactorrhea, gynecological bleeding, hallucinations, hyperglycemia, hyperprolactinemia, hypersensitivity, hypertensive crisis, muscle spasm, rash, restless legs syndrome, seizures upon treatment discontinuation, supraventricular arrhythmia, tinnitus (upon treatment discontinuation), trismus, and urticaria.
-
7 DRUG INTERACTIONS
7.1 Drugs Having Clinically Important Interactions with DRIZALMA SPRINKLE
Table 7: Clinically Important Drug InteractionsMonoamine Oxidase Inhibitors (MAOIs)
Clinical Impact
Concomitant use of SSRIs and SNRIs including duloxetine with MAOIs increases the risk of serotonin syndrome.
Intervention
•The use of MAOIs intended to treat psychiatric disorders with duloxetine or within 5 days of stopping treatment with duloxetine is contraindicated [see Contraindications (4) and Warnings and Precautions (5.4)].•The use of duloxetine delayed-release capsules within 14 days of stopping an MAOI intended to treat psychiatric disorders is also contraindicated [see Contraindications (4) and Warnings and Precautions (5.4)].•Starting duloxetine in a patient who is being treated with MAOIs is also contraindicated [see Dosage and Administration (2.10), Contraindications (4), Warnings and Precautions (5.4)]. Examples
Selegiline, tranylcypromine, isocarboxazid, phenelzine, linezolid, intravenous methylene blue
Other Serotonergic Drugs
Clinical Impact
Concomitant use of duloxetine with other serotonergic drugs increases the risk of serotonin syndrome.
Intervention
•Patients should be made aware of a potential increased risk for serotonin syndrome, particularly during treatment initiation and dose increases.•Monitor for symptoms of serotonin syndrome when duloxetine is used concomitantly with other drugs that may affect the serotonergic neurotransmitter systems.•Treatment with duloxetine delayed-release capsules and any concomitant serotonergic agents, should be discontinued immediately if the above events occur and supportive symptomatic treatment should be initiated [see Warnings and Precautions (5.4)]. Examples
Other SNRIs, SSRIs, triptans, tricyclic antidepressants, opioids, lithium, tryptophan, buspirone, amphetamines, and St. John’s Wort
Inhibitors of CYP1A2
Clinical Impact
Concomitant use of duloxetine with CYP1A2 inhibitors increases AUC, Cmax, t1/2 of duloxetine.
Intervention
Avoid concomitant use of duloxetine delayed-release capsules with potent CYP1A2 inhibitors [see Warnings and Precautions (5.12), Clinical Pharmacology (12.3)].
Examples
Fluvoxamine, cimetidine, ciprofloxacin, enoxacin
Dual Inhibition of CYP1A2 and CYP2D6
Clinical Impact
Concomitant administration of duloxetine with potent CYP1A2 inhibitors to CYP2D6 poor metabolizers results in increased AUC and Cmax of duloxetine.
Intervention
Avoid co-administration of duloxetine delayed-release capsules and potent CYP1A2 inhibitors to CYP2D6 poor metabolizers [see Clinical Pharmacology (12.3)].
Examples
Fluvoxamine, cimetidine, ciprofloxacin, enoxacin
Drugs that Interfere with Hemostasis
Clinical Impact
Concomitant use of duloxetine with an antiplatelet or anticoagulant drug may potentiate the risk of bleeding.
Intervention
Closely monitor for bleeding for patients receiving an antiplatelet or anticoagulant drug when duloxetine is initiated or discontinued [Warnings and Precaution (5.5)].
Examples
NSAIDs, aspirin, warfarin
Inhibitors of CYP2D6
Clinical Impact
Concomitant use of duloxetine with CYP2D6 inhibitors increase AUC of duloxetine. Greater degrees of inhibition are expected with higher doses of CYP2D6 inhibitors.
Intervention
Exercise caution when co-administering duloxetine delayed-release capsules and potent CYP2D6 inhibitors [see Warnings and Precautions (5.12), Clinical Pharmacology (12.3)].
Examples
Paroxetine, fluoxetine, quinidine
Drugs Metabolized by CYP2D6
Clinical Impact
Concomitant use of duloxetine increases AUC of a drug primarily metabolized by CYP2D6 which may increase the risk of toxicity of the CYP2D6 substrate drug.
Intervention
Monitor plasma concentrations of CYP2D6 substrate and reduce dosage of CYP2D6 substrate drug if necessary [see Warnings and Precautions (5.12), Clinical Pharmacology (12.3)].
Examples
TCAs (nortriptyline, amitriptyline, imipramine, desipramine); phenothiazines (thioridazine); Type 1C antiarrhythmics (propafenone, flecainide)
Drugs that Affect Gastric Acidity
Clinical Impact
In patients with conditions that may slow gastric emptying (e.g., some diabetics) and drugs that raise the gastrointestinal pH may lead to earlier the release of duloxetine.
Intervention
Use with caution [see Clinical Pharmacology (12.3)].
Examples
Aluminum-and magnesium-containing antacids, famotidine, proton pump inhibitors
Drugs Metabolized by CYP1A2
Clinical Impact
Concomitant use of duloxetine with CYP1A2 substrates may increase the AUC of CYP1A2 substrate.
Intervention
Use with caution [see Clinical Pharmacology (12.3)].
Examples
Theophylline, caffeine
CNS Drugs
Clinical Impact
Concomitant use of duloxetine with other centrally acting drugs may increase the CNS effects of duloxetine.
Intervention
Use with caution [see Warnings and Precautions (5.12)].
Examples
Centrally acting CNS drugs
Drugs Highly Bound to Plasma Protein
Clinical Impact
Concomitant use of duloxetine with highly protein bound drugs may cause increased free concentrations of the other drug, potentially resulting in adverse reactions.
Intervention
Use with caution [see Clinical Pharmacology (12.3)].
Examples
Highly plasma protein binding drugs
Alcohol
Clinical Impact
Concomitant use of duloxetine and alcohol may cause liver injury or aggravate pre-existing liver disease.
Intervention
Avoid use patients with chronic liver disease or heavy alcohol use [see Dosage and Administration (2.7), Warnings and Precautions (5.2, 5.12)].
-
8 USE IN SPECIFIC POPULATIONS
8.1 Pregnancy
Pregnancy Exposure Registry
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to antidepressants during pregnancy. Healthcare providers are encouraged to register patients by calling the National Pregnancy Registry for Antidepressants at 1-844-405-6185 or visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/
Risk Summary
Based on data from published observational studies, exposure to SNRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Warnings and Precautions (5.5) and Clinical Considerations].
Data from a postmarketing retrospective cohort study indicate that use of duloxetine in the month before delivery may be associated with an increased risk of postpartum hemorrhage. Data from published literature and from a postmarketing retrospective cohort study have not identified a clear drug-associated risk of major birth defects or other adverse developmental outcomes (see Data). There are risks associated with untreated depression in pregnancy and with exposure to SNRIs and SSRIs, including DRIZALMA SPRINKLE, during pregnancy (see Clinical Considerations).
In rats and rabbits treated with duloxetine during the period of organogenesis, fetal weights were decreased but there was no evidence of developmental effects at doses up to 3 and 6 times, respectively, the maximum recommended human dose (MRHD) of 120 mg/day given to adolescents on a mg/m2 basis. When duloxetine was administered orally to pregnant rats throughout gestation and lactation, pup weights at birth and pup survival to 1 day postpartum were decreased at a dose 2 times the MRHD given to adolescents on a mg/m2 basis. At this dose, pup behaviors consistent with increased reactivity, such as increased startle response to noise and decreased habituation of locomotor activity were observed. Post-weaning growth was not adversely affected.
The estimated of background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
Clinical Considerations
Disease-associated Maternal and/or Embryo/Fetal Risk
Women who discontinued antidepressants during pregnancy are more likely to experience a relapse of major depression than women who continued antidepressants. This finding is from a prospective, longitudinal study that followed 201 pregnant women with a history of major depressive disorder who were euthymic and taking antidepressants at the beginning of pregnancy. Consider the risk of untreated depression when discontinuing or changing treatment with antidepressant medication during pregnancy and the postpartum.
Pregnant women with fibromyalgia are at increased risk for adverse maternal and infant outcomes including preterm premature rupture of membranes, preterm birth, small for gestational age, intrauterine growth restriction, placental disruption, and venous thrombosis. It is not known if these adverse maternal and fetal outcomes are a direct result of fibromyalgia or other comorbid factors.
Maternal Adverse Reactions
Use of DRIZALMA SPRINKLE in the month before delivery may be associated with an increased risk of postpartum hemorrhage [see Warnings and Precautions (5.5)].
Fetal/Neonatal Adverse Reaction
Neonates exposed to duloxetine and other SNRIs or SSRIs late in the third trimester have developed complications requiring prolonged hospitalization, respiratory support, and tube feeding. Such complications can arise immediately upon delivery. Reported clinical findings have included respiratory distress, cyanosis, apnea, seizures, temperature instability, feeding difficulty, vomiting, hypoglycemia, hypotonia, hypertonia, hyperreflexia, tremor, jitteriness, irritability, and constant crying. These findings are consistent with either a direct toxic effect of the SNRIs or SSRIs, or possibly, a drug discontinuation syndrome. It should be noted that, in some cases, the clinical picture is consistent with serotonin syndrome [see Warnings and Precautions (5.4)].
Data
Human Data
Data from a postmarketing retrospective claims-based cohort study found an increased risk for postpartum hemorrhage among 955 pregnant women exposed to duloxetine in the last month of pregnancy compared to 4,128,460 unexposed pregnant women (adjusted relative risk: 1.53; 95% CI: 1.08-2.18). The same study did not find a clinically meaningful increase in the risk for major birth defects in the comparison of 2532 women exposed to duloxetine in the first trimester of pregnancy to 1,284,827 unexposed women after adjusting for several confounders. Methodologic limitations include possible residual confounding, misclassification of exposure and outcomes, lack of direct measures of disease severity, and lack of information about alcohol use, nutrition, and over-the-counter medication exposures.
Animal Data
In animal reproduction studies, duloxetine has been shown to have adverse effects on embryo/fetal and postnatal development.
When duloxetine was administered orally to pregnant rats and rabbits during the period of organogenesis, there was no evidence of malformations or developmental variations at doses up to 45 mg/kg/day (3 and 6 times, respectively, the MRHD of 120 mg/day given to adolescents on a mg/m2 basis. However, fetal weights were decreased at this dose, with a no-effect dose of 10 mg/kg/day (approximately equal to the MRHD in rats and 2 times the MRHD in rabbits).
When duloxetine was administered orally to pregnant rats throughout gestation and lactation, the survival of pups to 1 day postpartum and pup body weights at birth and during the lactation period were decreased at a dose of 30 mg/kg/day (2 times the MRHD given to adolescents on a mg/m2 basis); the no-effect dose was 10 mg/kg/day. Furthermore, behaviors consistent with increased reactivity, such as increased startle response to noise and decreased habituation of locomotor activity, were observed in pups following maternal exposure to 30 mg/kg/day. Post-weaning growth and reproductive performance of the progeny were not affected adversely by maternal duloxetine treatment.
8.2 Lactation
Risk Summary
Data from the published literature report the presence of duloxetine in human milk (see Data). There are reports of sedation, poor feeding, and poor weight gain in infants exposed to duloxetine through breast milk (see Clinical Considerations). There are no data on the effect of duloxetine on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for DRIZALMA SPRINKLE and any potential adverse effects on the breastfed child from DRIZALMA SPRINKLE or from the underlying maternal condition.
Clinical Considerations
Infants exposed to DRIZALMA SPRINKLE should be monitored for sedation, poor feeding and poor weight gain.
Data
Disposition of duloxetine was studied in 6 lactating women who were at least 12 weeks postpartum and had elected to wean their infants. The women were given 40 mg of duloxetine delayed-release capsules twice daily for 3.5 days. The peak concentration measured in breast milk occurred at a median of 3 hours after the dose. The amount of duloxetine in breast milk was approximately 7 mcg/day while on that dose; the estimated daily infant dose was approximately 2 mcg/kg/day, which is less than 1% of the maternal dose. The presence of duloxetine metabolites in breast milk was not examined.
8.4 Pediatric Use
The safety and effectiveness of DRIZALMA SPRINKLE have been established for treatment of generalized anxiety disorder (GAD) in pediatric patients ages 7 to 17 years of age. The safety and effectiveness of DRIZALMA SPRINKLE have not been established in pediatric patients with major depressive disorder (MDD), diabetic peripheral neuropathic pain, or chronic musculoskeletal pain.
Antidepressants increased the risk of suicidal thoughts and behavior in pediatric patients. Monitor all pediatric patients being treated with antidepressants for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of treatment, or at times of dosage changes [see Warnings and Precautions (5.1)]. Perform regular monitoring of weight and growth in pediatric patients treated with DRIZALMA SPRINKLE [see Adverse Reactions (6.1)].
Generalized Anxiety Disorder
Use of DRIZALMA SPRINKLE for the treatment of GAD in patients 7 to 17 years of age is supported by one 10-week, placebo- controlled trial (GAD-6). The study included 272 pediatric patients with GAD of which 47% were 7 to 11 years of age (53% were 12 to 17 years of age). Duloxetine delayed-release capsules demonstrated superiority over placebo as measured by greater improvement in the Pediatric Anxiety Rating Scale (PARS) for GAD severity score [see Clinical Studies (14.3)].
The safety and effectiveness of DRIZALMA SPRINKLE for the treatment of GAD in pediatric patients less than 7 years of age have not been established.
Fibromyalgia
The safety and effectiveness of DRIZALMA SPRINKLE for the treatment of fibromyalgia in patients less than 13 years of age have not been established.
Additional pediatric use information is approved for Eli Lilly and Company, Inc.’s CYMBALTA (duloxetine delayed-release capsules). However, due to Eli Lilly and Company Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
Major Depressive Disorder
The safety and effectiveness of DRIZALMA SPRINKLE have not been established in pediatric patients for the treatment of MDD. Efficacy of duloxetine delayed-release capsules was not demonstrated in two 10 week, placebo-controlled trials with 800 pediatric patients with MDD, aged 7 to 17 years old with MDD. Neither duloxetine delayed-release capsules nor an active control (approved for treatment of pediatric MDD) was superior to placebo.
The most frequently observed adverse reactions in the clinical trials included nausea, headache, decreased weight, and abdominal pain. Decreased appetite and weight loss have been observed in association with the use of SSRIs and SNRIs. Juvenile Animal Toxicology Data
Duloxetine administration to young rats from post-natal day 21 (weaning) through post-natal day 90 (adult) resulted in decreased body weights that persisted into adulthood, but recovered when drug treatment was discontinued; slightly delayed (~1.5 days) sexual maturation in females, without any effect on fertility; and a delay in learning a complex task in adulthood, which was not observed after drug treatment was discontinued. These effects were observed at the high dose of 45 mg/kg/day (2 times the MRHD, for a child); the no-effect-level was 20 mg/kg/day (≈1 times the MRHD, for a child).
8.5 Geriatric Use
Geriatric Exposure in Premarketing Clinical Trials of duloxetine delayed-release capsules
•Of the 2,418 patients in MDD trials, 6% (143) were 65 years of age or over.•Of the 1041 patients in CLBP trials, 21% (221) were 65 years of age or over.•Of the 487 patients in OA trials, 41% (197) were 65 years of age or over.•Of the 1,074 patients in the DPNP trials, 33% (357) were 65 years of age or over.•Of the 1,761 patients in FM trials, 8% (140) were 65 years of age or over.
In the MDD, GAD, DPNP, FM, OA, and CLBP studies, no overall differences in safety or effectiveness were generally observed between these patients and younger adult patients, and other reported clinical experience has not identified differences in responses between these geriatric and younger adult patients, but greater sensitivity of some older patients cannot be ruled out.
SSRIs and SNRIs, including duloxetine delayed-release capsules have been associated with clinically significant hyponatremia in geriatric patients, who may be at greater risk for this adverse reaction [see Warnings and Precautions (5.13)].
In an analysis of data from all placebo-controlled-trials, patients treated with duloxetine delayed-release capsules reported a higher rate of falls compared to patients treated with placebo. The increased risk appears to be proportional to a patient’s underlying risk for falls. Underlying risk appears to increase steadily with age. As geriatric patients tend to have a higher prevalence of risk factors for falls such as medications, medical comorbidities and gait disturbances, the impact of increasing age by itself on falls during treatment with duloxetine delayed-release capsules is unclear. Falls with serious consequences including bone fractures and hospitalizations have been reported with duloxetine delayed-release capsules use [see Warnings and Precautions (5.3) and Adverse Reactions (6.1)].
The pharmacokinetics of duloxetine after a single dose of 40 mg were compared in healthy elderly females (65 to 77 years) and healthy middle-age females (32 to 50 years). There was no difference in the Cmax, but the AUC of duloxetine was somewhat (about 25%) higher and the half-life about 4 hours longer in the elderly females. Population pharmacokinetic analyses suggest that the typical values for clearance decrease by approximately 1% for each year of age between 25 to 75 years of age; but age as a predictive factor only accounts for a small percentage of between-patient variability. Dosage adjustment based on the age of the adult patient is not necessary.
8.6 Gender
Duloxetine’s half-life is similar in men and women. Dosage adjustment based on gender is not necessary.
8.7 Smoking Status
Duloxetine bioavailability (AUC) appears to be reduced by about one-third in smokers. Dosage modifications are not recommended for smokers.
8.9 Hepatic Impairment
Patients with clinically evident hepatic impairment have decreased duloxetine metabolism and elimination. After a single 20 mg dose of duloxetine delayed-release capsules, 6 cirrhotic patients with moderate liver impairment (Child-Pugh Class B) had a mean plasma duloxetine clearance about 15% that of age- and gender-matched healthy subjects, with a 5 fold increase in mean exposure (AUC). Although Cmax was similar to normals in the cirrhotic patients, the half-life was about 3 times longer [see Dosage and Administration (2.8) and Warnings and Precautions (5.14)].
8.10 Severe Renal Impairment
Limited data are available on the effects of duloxetine in patients with end-stage renal disease (ESRD). After a single 60 mg dose of duloxetine, Cmax and AUC values were approximately 100% greater in patients with end-stage renal disease receiving chronic intermittent hemodialysis than in subjects with normal renal function. The elimination half-life, however, was similar in both groups. The AUCs of the major circulating metabolites, 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate, largely excreted in urine, were approximately 7 to 9 fold higher and would be expected to increase further with multiple dosing. Population PK analyses suggest that mild to moderate degrees of renal impairment (estimated CrCl 30 to 80 mL/min) have no significant effect on duloxetine apparent clearance [see Dosage and Administration (2.8) and Warnings and Precautions (5.14)].
-
9 DRUG ABUSE AND DEPENDENCE
9.2 Abuse
In animal studies, duloxetine did not demonstrate barbiturate-like (depressant) abuse potential.
While duloxetine delayed-release capsules have not been systematically studied in humans for its potential for abuse, there was no indication of drug-seeking behavior in the clinical trials. However, it is not possible to predict on the basis of premarketing experience the extent to which a CNS active drug will be misused, diverted, and/or abused once marketed. Consequently, physicians should carefully evaluate patients for a history of drug abuse and follow such patients closely, observing them for signs of misuse or abuse of duloxetine delayed-release capsules (e.g., development of tolerance, incrementation of dose, drug-seeking behavior).
-
10 OVERDOSAGE
10.1 Signs and Symptoms
In postmarketing experience, fatal outcomes have been reported for acute overdoses, primarily with mixed overdoses, but also with duloxetine only, at doses as low as 1000 mg. Signs and symptoms of overdose (duloxetine alone or with mixed drugs) included somnolence, coma, serotonin syndrome, seizures, syncope, tachycardia, hypotension, hypertension, and vomiting.
10.2 Management of Overdose
There is no specific antidote to a duloxetine overdosage, but if serotonin syndrome ensues, specific treatment (such as with cyproheptadine and/or temperature control) may be considered.
In case of acute overdose, treatment should consist of those general measures employed in the management of overdose with any drug, such as assuring an an adequate airway, oxygenation, and ventilation and monitoring cardiac rhythm and vital signs. Gastric lavage with a large-bore orogastric tube with appropriate airway protection, if needed, may be indicated if performed soon after ingestion or in symptomatic patients. Induction of emesis is not recommended.
Activated charcoal may be useful in limiting absorption of duloxetine from the gastrointestinal tract. Administration of activated charcoal has been shown to decrease AUC and Cmax by an average of one-third, although some patients had a limited effect of activated charcoal. Due to the large volume of distribution of duloxetine, forced diuresis, dialysis, hemoperfusion, and exchange transfusion are unlikely to be beneficial.
In managing overdose, the possibility of multiple drug involvement should be considered. A specific caution involves patients who overdose with duloxetine delayed-release capsules and tricyclic antidepressants. In such a case, decreased clearance of the parent tricyclic and/or its active metabolite may increase the possibility of clinically significant sequelae and extend the time needed for close medical observation [see Warnings and Precautions (5.4) and Drug Interactions (7)]. In case of an overdose, consult a Certified Poison Control Center (1-800-222-1222 or www.poison.org) for up-to-date guidance and advice.
-
11 DESCRIPTION
Duloxetine hydrochloride is a selective serotonin and norepinephrine reuptake inhibitor (SNRI). The chemical name of duloxetine hydrochloride is (+)-(S)-N-methyl-γ-(1-naphthyloxy)-2-thiophenepropylamine hydrochloride. The molecular formula is C18H19NOS•HCl, which corresponds to a molecular weight of 333.88. The structural formula is:
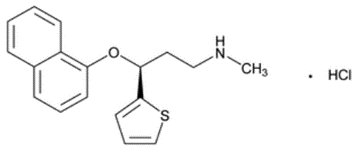
Duloxetine hydrochloride, USP is a white to off-white powder, which is freely soluble in methanol, soluble in dichloromethane, and slightly soluble in water. The molecular formula of duloxetine free base is C18H19NOS and its molecular weight is 297.38.
Each DRIZALMA SPRINKLE (duloxetine delayed-release capsule) for oral administration contains enteric-coated pellets containing a total of 22.4 mg, 33.6 mg, 44.9 mg or 67.3 mg of duloxetine hydrochloride, equivalent to 20 mg, 30 mg, 40 mg or 60 mg of duloxetine free base, respectively. These enteric-coated pellets are designed to prevent degradation of the drug in the acidic environment of the stomach. Inactive ingredients of the pellets include ascorbic acid, hypromellose, hypromellose phthalate, polyethylene glycol, starch, sucrose, sugar spheres, talc, titanium dioxide, and triethyl citrate. The capsule shell ingredients for 20 mg strength are D&C Yellow 10, FD &C Blue 1, FD &C Red 40, gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 30 mg strength are FD &C Blue 1, FD &C Red 40 and FD &C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 40 mg strength are gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 60 mg strength are D&C Yellow 10 (present in body), FD &C Blue 1, FD &C Red 40, FD &C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide.
The imprinting ink for 20 mg, 30 mg, 40 mg, and 60 mg strength capsules was made of ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol and shellac.
DRIZALMA SPRINKLE does not comply with the USP dissolution test.
-
12 CLINICAL PHARMACOLOGY
12.1 Mechanism of Action
Although the exact mechanisms of the antidepressant, central pain inhibitory and anxiolytic actions of duloxetine in humans are unknown, these actions are believed to be related to its potentiation of serotonergic and noradrenergic activity in the CNS.
12.2 Pharmacodynamics
Preclinical studies have shown that duloxetine is a potent inhibitor of neuronal serotonin and norepinephrine reuptake and a less potent inhibitor of dopamine reuptake. Duloxetine has no significant affinity for dopaminergic, adrenergic, cholinergic, histaminergic, opioid, glutamate, and GABA receptors in vitro. Duloxetine does not inhibit monoamine oxidase (MAO).
Duloxetine is in a class of drugs known to affect urethral resistance [see Warnings and Precautions (5.15)].
Cardiac Electrophysiology
The effect of duloxetine delayed-release capsules 160 mg and 200 mg administered twice daily (2.7 and 3.3 times the maximum recommended dosage, respectively) to steady state was evaluated in a randomized, double-blinded, two-way crossover study in 117 healthy female adult subjects. No QT interval prolongation was detected. Duloxetine delayed-release capsules appears to be associated with concentration-dependent but not clinically meaningful QT shortening.
12.3 Pharmacokinetics
Duloxetine hydrochloride pharmacokinetics are dose proportional over the therapeutic range. Steady-state plasma concentrations are typically achieved after 3 days of dosing.
Absorption
After oral duloxetine delayed-release capsules administration, duloxetine hydrochloride is well absorbed. Peak plasma concentration of duloxetine is attained at 5 hours following administration of duloxetine delayed-release capsules. There is a 3 hour delay in absorption and a one- third increase in apparent clearance of duloxetine after an evening dose as compared to a morning dose.
Effect of food
Compared to fasted state administration, high-fat, high-calorie meal (containing approximately 150 kcal from protein, 250 kcal from carbohydrates and 500 kcal from fat) did not have a significant effect on Cmax and AUC of duloxetine hydrochloride. However, Tmax of duloxetine hydrochloride delayed by approximately 1.7 hours with the administration of high-fat, high-calorie meal.
Duloxetine delayed-release capsules administered under fasting conditions to healthy adults by sprinkling the entire contents on one-tablespoon (15 mL) of applesauce did not significantly affect Tmax, Cmax, and AUC of duloxetine.
Distribution
The apparent volume of distribution averages about 1640 L. Duloxetine is highly bound (>90%) to proteins in human plasma, binding primarily to albumin and α1-acid glycoprotein. The interaction between duloxetine and other highly protein bound drugs has not been fully evaluated. Plasma protein binding of duloxetine is not affected by renal or hepatic impairment.
Elimination
Metabolism
Biotransformation and disposition of duloxetine in humans have been determined following oral administration of 14C-labeled duloxetine. Duloxetine comprises about 3% of the total radiolabeled material in the plasma, indicating that it undergoes extensive metabolism to numerous metabolites. The major biotransformation pathways for duloxetine involve oxidation of the naphthyl ring followed by conjugation and further oxidation. Both CYP1A2 and CYP2D6 catalyze the oxidation of the naphthyl ring in vitro. Metabolites found in plasma include 4-hydroxy duloxetine glucuronide and 5-hydroxy, 6-methoxy duloxetine sulfate. Duloxetine undergoes extensive metabolism, but the major circulating metabolites have not been shown to contribute significantly to the pharmacologic activity of duloxetine.
Excretion
Only trace (<1% of the dose) amounts of unchanged duloxetine are present in the urine. Most (about 70%) of the duloxetine dose appears in the urine as metabolites of duloxetine; about 20% is excreted in the feces. Mean terminal half-life was 12.4 hours (range 7.8 to 22.2 hour) in subjects receiving a single dose DRIZALMA SPRINKLE.
Specific Populations
Pediatric Patients
Duloxetine steady-state plasma concentration was comparable in pediatric patients7 to 17 years of age) and adult patients. The average steady-state duloxetine concentration was approximately 30% lower in the pediatric population relative to the adults. The model-predicted duloxetine steady state plasma concentrations in pediatric patients 7 to 17 years of age were mostly within the concentration range observed in adult patients and did not exceed the concentration range in adults.
Drug Interaction Studies
Clinical Studies: Effect of other drugs on duloxetine
Fluvoxamine:
When duloxetine 60 mg was co-administered with fluvoxamine 100 mg, a potent CYP1A2 inhibitor, to male subjects (n = 14) duloxetine AUC was increased approximately 6 fold, the Cmax was increased about 2.5 fold, and duloxetine t1/2 was increased approximately 3 fold [see Dosage and Administration (2.6) and Drug Interactions (7.1)].
Fluvoxamine in CYP2D6 poor metabolizer subjects:
Concomitant administration of duloxetine 40 mg twice daily with fluvoxamine 100 mg, a potent CYP1A2 inhibitor, to CYP2D6 poor metabolizer subjects (n = 14) resulted in a 6 fold increase in duloxetine AUC and Cmax[see Dosage and Administration (2.6) and Drug Interactions (7.1)].
Paroxetine:
Concomitant use of duloxetine (40 mg once daily) with paroxetine (20 mg once daily), a strong CYP2D6 inhibitor, increased the concentration of duloxetine AUC by about 60%, and greater degrees of inhibition are expected with higher doses of paroxetine [see Drug Interactions (7.1)].
Lorazepam:
Under steady-state conditions for duloxetine (60 mg Q 12 hours) and lorazepam (2 mg Q 12 hours), the pharmacokinetics of duloxetine were not affected by co-administration.
Temazepam:
Under steady-state conditions for duloxetine (20 mg qhs) and temazepam (30 mg qhs), the pharmacokinetics of duloxetine were not affected by co-administration.
Drugs that Affect Gastric Acidity:
Co-administration of duloxetine delayed-release capsules with aluminum- and magnesium-containing antacids (51 mEq) or duloxetine delayed-release capsules with famotidine, had no significant effect on the rate or extent of duloxetine absorption after administration of a 40 mg oral dose [see Drug Interactions (7.1)].
Clinical Studies: Effect of duloxetine on other drugs
Theophylline:
In two clinical studies the average (90% confidence interval) increase in theophylline (a CYP1A2 substrate) AUC was 7% (1% to 15%) and 20% (13% to 27%) when co-administered with duloxetine (60 mg twice daily) [see Drug Interactions (7.1)].
Desipramine:
When duloxetine was administered (at a dose of 60 mg twice daily) in conjunction with a single 50 mg dose of desipramine, a CYP2D6 substrate, the AUC of desipramine increased 3 fold [see Drug Interactions (7.1)].
Warfarin:
Under steady-state condition for warfarin (2 to 9 mg once daily) and duloxetine (60 or 120 mg once daily), the total warfarin (protein bound plus free drug) pharmacokinetics (AUCτ,ss, Cmax,ss or tmax,ss) for both R- and S-warfarin (a CYP2C9 substrate) were not altered by duloxetine [see Drug Interactions (7.1)].
In vitro Studies
Results of in vitro studies demonstrate that duloxetine does not inhibit activity of drugs metabolized by CYP3A (e.g., oral contraceptives and other steroidal agents) and drugs metabolized by CYP2C19.
Alcohol:
An in vitro study showed significant increases of duloxetine hydrochloride release from DRIZALMA SPRINKLE at 2 hours to approximately 86% and 56% of the drug release in the presence of 40% and 20% alcohol, respectively. Effect of 5% alcohol on drug release was not observed at 2 hours. There is no in vivo study conducted for the effect of alcohol on drug exposure.
-
13 NONCLINICAL TOXICOLOGY
13.1 Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis - Duloxetine was administered in the diet to mice and rats for 2 years.
In female mice receiving duloxetine at 140 mg/kg/day (3 times the MRHD of 120 mg/day given to children on a mg/m2 basis), there was an increased incidence of hepatocellular adenomas and carcinomas. The no-effect dose was 50 mg/kg/day (1 times the MRHD given to children). Tumor incidence was not increased in male mice receiving duloxetine at doses up to 100 mg/kg/day (2 times the MRHD given to children).
In rats, dietary doses of duloxetine up to 27 mg/kg/day in females (1 times the MRHD given to children) and up to 36 mg/kg/day in males (1.4 times the MRHD given to children) did not increase the incidence of tumors.
Mutagenesis - Duloxetine was not mutagenic in the in vitro bacterial reverse mutation assay (Ames test) and was not clastogenic in an in vivo chromosomal aberration test in mouse bone marrow cells. Additionally, duloxetine was not genotoxic in an in vitro mammalian forward gene mutation assay in mouse lymphoma cells or in an in vitro unscheduled DNA synthesis (UDS) assay in primary rat hepatocytes, and did not induce sister chromatid exchange in Chinese hamster bone marrow in vivo.
Impairment of Fertility - Duloxetine administered orally to either male or female rats prior to and throughout mating at doses up to 45 mg/kg/day (3 times the MRHD given to adolescents on a mg/m2 basis) did not alter mating or fertility.
-
14 CLINICAL STUDIES
The efficacy of duloxetine delayed-release capsules has been established in the following populations in adequate and well-controlled trials:
- Major Depressive Disorder (MDD): 4 short-term and 1 maintenance trial in adults [see Clinical Studies (14.1)].
- Generalized Anxiety Disorder (GAD): 3 short-term trials in adults, 1 maintenance trial in adults, and 1 short-term trial in pediatric patients 7 to 17 years of age [see Clinical Studies (14.2)].
- Diabetic Peripheral Neuropathic Pain (DPNP): Two 12 week trials in adults [see Clinical Studies (14.3)].
- Fibromyalgia (FM): Two trials in adults (one of 3 months duration and one of 6 months duration) [see Clinical Studies (14.4)].
- Chronic Musculoskeletal Pain: Two 12 to 13 week trials in adult patients with chronic low back pain (CLBP) and one 13 week trial in adult patients with chronic pain due to osteoarthritis [see Clinical Studies (14.5)].
Additionally, a summary of the following trials that did not demonstrate efficacy are presented below: Study FM-3 (a 16-week trial in adult patients with fibromyalgia), Study CLBP-2 (a 13-week trial in adult patients with CLBP), and Study OA-2 (a 13-week trial in adult patients with chronic pain due to OA).
14.1 Major Depressive Disorder
The efficacy of duloxetine delayed-release capsules as a treatment for MDD was established in 4 randomized, double-blind, placebo-controlled, fixed-dose trials in adult outpatients (18 to 83 years) meeting DSM-IV criteria for major depression. In Studies 1 and 2 studies, patients were randomized to duloxetine delayed-release capsules 60 mg once daily (N = 123 and N = 128, respectively) or placebo (N = 122 and N = 139, respectively) for 9 weeks; in Study 3, patients were randomized to duloxetine delayed-release capsules 20 or 40 mg twice daily (N = 86 and N = 91, respectively) or placebo (N = 89) for 8 weeks; in Study 4, patients were randomized to duloxetine delayed-release capsules 40 or 60 mg twice daily (N = 95 and N = 93, respectively) or placebo (N = 93) for 8 weeks. There is no evidence that doses greater than 60 mg/day confer additional benefits.
In all 4 trials, duloxetine delayed-release capsules demonstrated superiority over placebo as measured by improvement in the 17-item Hamilton Depression Rating Scale (HAMD-17) total score (Studies 1-4 in Table 8).
In all of these clinical trials, analyses of the relationship between treatment outcome and age, gender, and race did not suggest any differential responsiveness on the basis of these patient characteristics.
Table 8: Summary of the Primary Efficacy Results for Adult Trials in MDD
Study Number
Treatment Group
Primary Efficacy Measure: HAMD-17
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Differencea(95% CI)
Study 1
Duloxetine delayed-release (60 mg/day)b
Placebo
21.5 (4.10)
21.1 (3.71)
-10.9 (0.70)
-6.1 (0.69)
-4.9 (-6.8, -2.9)
-
Study 2
Duloxetine delayed-release (60 mg/day)b
Placebo
20.3 (3.32)
20.5 (3.42)
-10.5 (0.71)
-8.3 (0.67)
-2.2 (-4.0, -0.3)
-
Study 3
Duloxetine delayed-release (20 mg BID)b
Duloxetine delayed-release (40 mg BID)b
Placebo
18.6 (5.85)
18.1 (4.52)
17.2 (5.11)
-7.4 (0.80)
-8.6 (0.81)
-5.0 (0.81)
-2.4 (-4.7, -0.2)
-3.6 (-5.9, -1.4)
-
Study 4
Duloxetine delayed-release (40 mg BID)b
Duloxetine delayed-release (60 mg BID)b
Placebo
19.9 (3.54)
20.2 (3.41)
19.9 (3.58)
-11.0 (0.49)
-12.1 (0.49)
-8.8 (0.50)
-2.2 (-3.6, -0.9)
-3.3 (-4.7, -1.9)
-
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiplicity in trials where multiple dose groups were included.
a Difference (drug minus placebo) in least-squares mean change from baseline.
b Doses statistically significantly superior to placebo.
In another trial (Study 5), 533 patients meeting DSM-IV criteria for MDD received duloxetine delayed-release capsules 60 mg once daily during an initial 12 week open-label treatment phase. Two hundred and seventy-eight patients who responded to open label treatment (defined as meeting the following criteria at weeks 10 and 12: a HAMD-17 total score ≤9, Clinical Global Impressions of Severity (CGI-S) ≤2, and not meeting the DSM-IV criteria for MDD) were randomly assigned to continuation of duloxetine delayed-release capsules at the same dose (N = 136) or to placebo (N = 142) for 6 months. Patients on duloxetine delayed-release capsules experienced a statistically significantly longer time to relapse of depression than did patients on placebo (Study 5 in Figure 1). Relapse was defined as an increase in the CGI-S score of ≥2 points compared with that obtained at week 12, as well as meeting the DSM-IV criteria for MDD at 2 consecutive visits at least 2 weeks apart, where the 2 week temporal criterion had to be satisfied at only the second visit.
Figure 1: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse
(MDD Study 5)
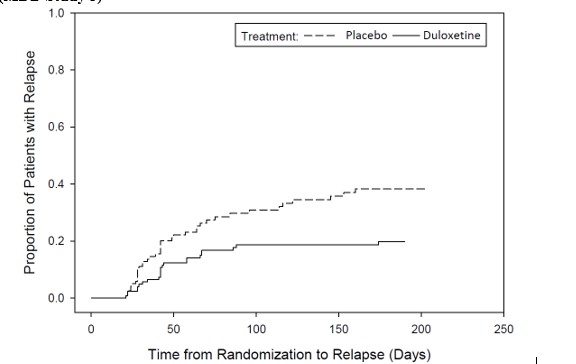
14.2 Generalized Anxiety Disorder
The efficacy of duloxetine delayed-release capsules in the treatment of generalized anxiety disorder (GAD) was established in 1 fixed-dose
The efficacy of duloxetine delayed-release capsules in the treatment of generalized anxiety disorder (GAD) was established in 1 fixed-dose randomized, double-blind, placebo-controlled trial (Study 1) and 2 flexible-dose randomized, double-blind, placebo-controlled trials Studies 2 and 3) in adult outpatients between 18 and 83 years of age meeting the DSM-IV criteria for GAD.
In 1 flexible-dose trial and in the fixed-dose trial, the starting dose was 60 mg once daily where down titration to 30 mg once daily was allowed for tolerability reasons before increasing it to 60 mg once daily. Fifteen percent of patients were down titrated. One flexible-dose study had a starting dose of 30 mg once daily for 1 week before increasing it to 60 mg once daily.
The 2 flexible-dose trials involved dose titration with duloxetine delayed-release capsules doses ranging from 60 mg once daily to 120 mg once daily (N = 168 and N = 162) compared to placebo (N = 159 and N = 161) over a 10 week treatment period. The mean dose for completers at endpoint in the flexible-dose studies was 104.75 mg/day. The fixed-dose study evaluated duloxetine delayed-release capsules doses of 60 mg once daily (N = 168) and 120 mg once daily (N = 170) compared to placebo (N = 175) over a 9 week treatment period. While a 120 mg/day dose was shown to be effective, there is no evidence that doses greater than 60 mg/day confer additional benefit.
In all 3 trials, duloxetine delayed-release capsules demonstrated superiority over placebo as measured by greater improvement in the Hamilton Anxiety Scale (HAM-A) total score (Studies 1-3 in Table 9) and by the Sheehan Disability Scale (SDS) global functional impairment score. The SDS is a composite measurement of the extent emotional symptoms disrupt patient functioning in 3 life domains: work/school, social life/leisure activities, and family life/home responsibilities.
In another trial (Study 4), 887 patients meeting DSM-IV-TR criteria for GAD received duloxetine delayed-release capsules 60 mg to 120 mg once daily during an initial 26 week open-label treatment phase. Four hundred and twenty-nine patients who responded to open-label treatment (defined as meeting the following criteria at weeks 24 and 26: a decrease from baseline HAM-A total score by at least 50% to a score no higher than 11, and a Clinical Global Impressions of Improvement [CGI-Improvement] score of 1 or 2) were randomly assigned to continuation of duloxetine delayed-release capsules at the same dose (N = 216) or to placebo (N = 213) and were observed for relapse. Of the patients randomized, 73% had been in a responder status for at least 10 weeks. Relapse was defined as an increase in CGI-Severity score at least 2 points to a score ≥4 and a MINI (Mini-International Neuropsychiatric Interview) diagnosis of GAD (excluding duration), or discontinuation due to lack of efficacy. Patients taking duloxetine delayed-release capsules experienced a statistically significantly longer time to relapse of GAD than did patients taking placebo (Study 4 in Figure 2).
Subgroup analyses did not indicate that there were any differences in treatment outcomes as a function of age or gender.
GAD Trial in Geriatric Patients
The efficacy of duloxetine delayed-release capsules in the treatment of patients ≥65 years of age with generalized anxiety disorder was established in one 10 week flexible-dose, randomized, double-blind, placebo-controlled trial (Study 5) in adults ≥65 years of age meeting the DSM-IV criteria for GAD. In this trial, the starting dose was 30 mg once daily for 2 weeks before further dose increases in 30 mg increments at treatment weeks 2, 4, and 7 up to 120 mg once daily were allowed based on investigator judgment of clinical response and tolerability. The mean dose for patients completing the 10 week acute treatment phase was 50.95 mg. Patients treated with duloxetine delayed-release capsules (N = 151) demonstrated significantly greater improvement compared with placebo (N = 140) on mean change from baseline to endpoint as measured by the Hamilton Anxiety Rating Scale total score (Study 5 in Table 9).
GAD Trial in Pediatric Patients 7 to 17 Years Old
The efficacy of duloxetine delayed-release capsules in the treatment of pediatric patients 7 to 17 years of age with generalized anxiety disorder (GAD) was established in 1 flexible-dose randomized, double-blind, placebo-controlled trial (Study 6) in pediatric outpatients with GAD (based on DSM-IV criteria).
In this trial, the starting dose was 30 mg once daily for 2 weeks. Further dose increases in 30 mg increments up to 120 mg once daily were allowed based on investigator judgment of clinical response and tolerability. The mean dose for patients completing the 10 week treatment phase was 57.6 mg/day. In this study, duloxetine delayed-release capsules (N = 135) demonstrated superiority over placebo (N = 137) from baseline to endpoint as measured by greater improvement in the Pediatric Anxiety Rating Scale (PARS) for GAD severity score (Study 6 in Table 9).
Table 9: Summary of the Primary Efficacy Results for GAD Trials
Study Number
Treatment Group
Primary Efficacy Measure
Mean Baseline Score (SD)
LS Mean Change from Baseline (SE)
Placebo-subtracted Differencea (95% CI)
Study 1
(HAM-A)
Duloxetine delayed-release (60 mg/day)b
25.1 (7.18)
-12.8 (0.68)
-4.4 (-6.2, -2.5)
Duloxetine delayed-release (120 mg/day)b
25.1 (7.24)
-12.5 (0.67)
-4.1 (-5.9, -2.3)
Placebo
25.8 (7.66)
-8.4 (0.67)
-
Study 2 (HAM-A)
Duloxetine delayed-release (60-120 mg/day)b
22.5 (7.44)
-8.1 (0.70)
-2.2 (-4.2, -0.3)
Placebo
23.5 (7.91)
-5.9 (0.70)
-
Study 3
(HAM-A)
Duloxetine delayed-release (60-120 mg/day)b
25.8 (5.66)
-11.8 (0.69)
-2.6 (-4.5, -0.7)
Placebo
25.0 (5.82)
-9.2 (0.67)
-
Study 5
(Geriatric) (HAM-A)
Duloxetine delayed-release (60-120 mg/day)b
24.6 (6.21)
-15.9 (0.63)
-4.2 (-5.9, -2.5)
Placebo
24.5 (7.05)
-11.7 (0.67)
-
Study 6
(Pediatric) (PARS for GAD)
Duloxetine delayed-release (30-120 mg/day)b
17.5 (1.98)
-9.7 (0.50)
-2.7 (-4.0, -1.3)
Placebo
17.4 (2.24)
-7.1 (0.50)
-
SD: standard deviation; SE: standard error; LS Mean: least-squares mean; CI: confidence interval, not adjusted for multiplicity in trials where multiple dose groups were included.
a Difference (drug minus placebo) in least-squares mean change from baseline.
b Dose statistically significantly superior to placebo.
Figure 2: Kaplan-Meier Estimation of Cumulative Proportion of Patients with Relapse (GAD Study 4)
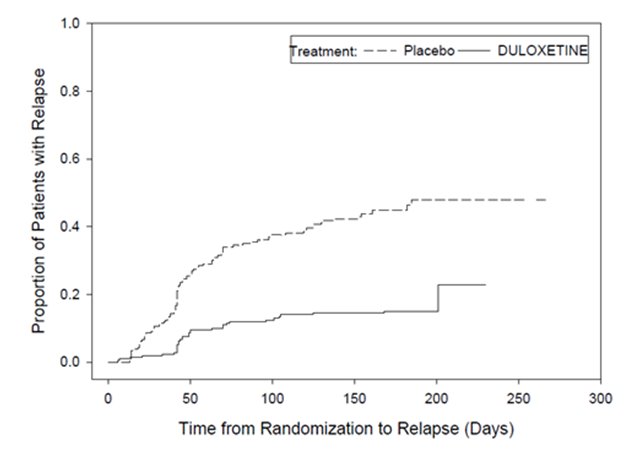
14.3 Diabetic Peripheral Neuropathic Pain in Adults
The efficacy of duloxetine delayed-release capsules for the management of neuropathic pain associated with diabetic peripheral neuropathy was established in 2 randomized, 12 week, double-blind, placebo-controlled, fixed-dose trials in adult patients having diabetic peripheral neuropathic pain for at least 6 months. Study DPNP-1 and Study DPNP-2 enrolled a total of 791 patients of whom 592 (75%) completed the studies. Patients enrolled had Type I or II diabetes mellitus with a diagnosis of painful distal symmetrical sensorimotor polyneuropathy for at least 6 months. The patients had a baseline pain score of ≥4 on an 11 point scale ranging from 0 (no pain) to 10 (worst possible pain). Patients were permitted up to 4 g of acetaminophen per day as needed for pain, in addition to duloxetine delayed-release capsules. Patients recorded their pain daily in a diary.
Both trials compared duloxetine delayed-release capsules 60 mg once daily or 60 mg twice daily with placebo. Study DPNP-1 additionally compared duloxetine delayed-release capsules 20 mg with placebo. A total of 457 patients (342 duloxetine delayed-release capsules, 115 placebo) were enrolled in Study DPNP-1 and a total of 334 patients (226 duloxetine delayed-release capsules, 108 placebo) were enrolled in Study DPNP-2. Treatment with duloxetine delayed-release capsules 60 mg one or two times a day statistically significantly improved the endpoint mean pain scores from baseline and increased the proportion of patients with at least a 50% reduction in pain scores from baseline. For various degrees of improvement in pain from baseline to study endpoint, Figures 3 and 4 show the fraction of patients achieving that degree of improvement. The figures are cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned 0% improvement. Some patients experienced a decrease in pain as early as week 1, which persisted throughout the trial.
Figure 3: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity – Study DPNP-1
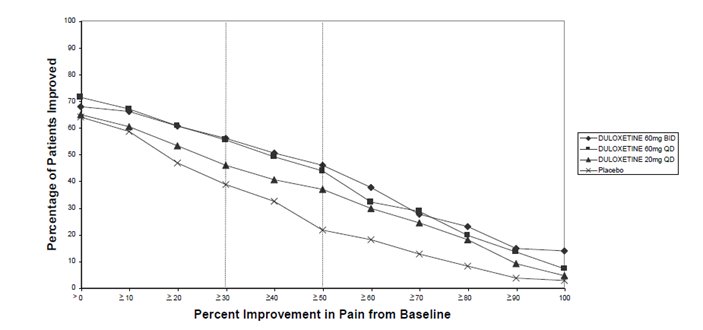
Figure 4: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity - DPNP-2
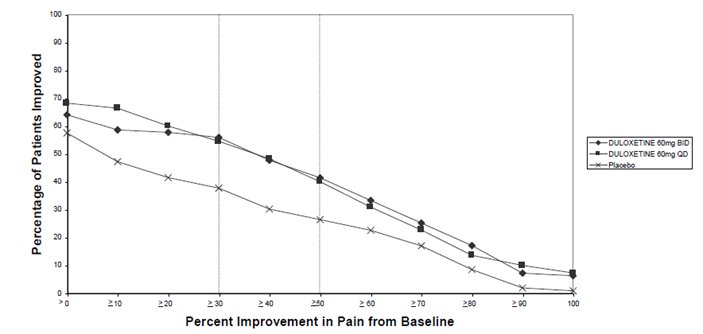
14.4 Fibromyalgia
The efficacy of duloxetine delayed-release capsules for the management of fibromyalgia in adults was established in two randomized, double-blind, placebo-controlled, fixed-dose trials in adult patients meeting the American College of Rheumatology criteria for fibromyalgia (a history of widespread pain for 3 months, and pain present at 11 or more of the 18 specific tender point sites). Study FM-1 was three months in duration and enrolled female patients only. Study FM-2 was six months in duration and enrolled male and female patients. Approximately 25% of participants had a comorbid diagnosis of MDD. Studies FM-1 and FM-2 enrolled a total of 874 patients of whom 541 (62%) completed the trials. A total of 354 patients (234 duloxetine, 120 placebo) were enrolled in Study FM-1 and a total of 520 patients (376 duloxetine, 144 placebo) were enrolled in Study FM-2 (5% male, 95% female). The patients had a baseline pain score of 6.5 on an 11-point scale ranging from 0 (no pain) to 10 (worse possible pain).
Both trials compared duloxetine 60 mg once daily or 120 mg daily (given in divided doses in Study FM-1 and as a single daily dose in Study FM-2) with placebo. Study FM-2 additionally compared duloxetine 20 mg with placebo during the initial three months of a six-month trial.
Treatment with duloxetine 60 mg or 120 mg daily statistically significantly improved the endpoint mean pain scores from baseline and increased the proportion of patients with at least a 50% reduction in pain score from baseline. Pain reduction was observed in patients both with and without comorbid MDD. However, the degree of pain reduction may be greater in patients with comorbid MDD. For various degrees of improvement in pain from baseline to study endpoint, Figures 5 and 6 show the fraction of patients achieving that degree of improvement in Studies FM-1 and FM-2, respectively. The figures are cumulative so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the trial were assigned 0% improvement. Some patients experienced a decrease in pain as early as week 1, which persisted throughout the trial. Improvement was also demonstrated on measures of function (Fibromyalgia Impact Questionnaires) and patient global impression of change (PGI). Neither trial demonstrated a benefit of 120 mg compared to 60 mg, and a higher dosage was associated with more adverse reactions and premature discontinuations of treatment.
Figure 5: Percentage of Adult Fibromyalgia Patients Achieving Various Levels of Pain Relief at Study Endpoint as Measured by 24-Hour Average Pain Severity (Study FM-1)
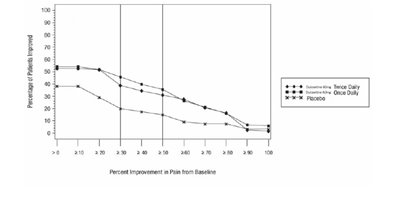
Figure 6: Percentage of Adult Fibromyalgia Patients Achieving Various Levels of Pain Relief at Study Endpoint as Measured by 24-Hour Average Pain Severity (Study FM-2)
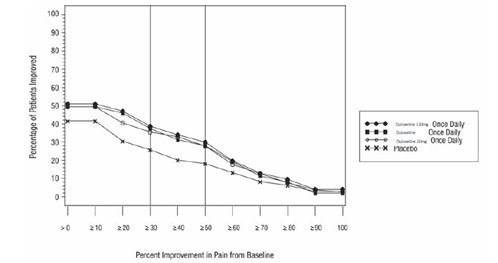
Additionally, the benefit of up-titration in non-responders to duloxetine at 60 mg/day was evaluated in a separate trial (Study FM-3). Adult patients were initially treated with duloxetine 60 mg once daily for eight weeks in open-label fashion. Subsequently, completers of this phase were randomized to double-blind treatment with duloxetine at either 60 mg once daily or 120 mg once daily. Responders were defined as patients who had at least a 30% reduction in pain score from baseline at the end of the 8-week treatment. Patients who were non-responders at 8 weeks were no more likely to meet response criteria at the end of 60 weeks of treatment if blindly titrated to duloxetine 120 mg as compared to those who were blindly continued on duloxetine 60 mg.
Additional pediatric use information is approved for Eli Lilly and Company, Inc.’s CYMBALTA (duloxetine delayed-release capsules). However, due to Eli Lilly and Company Inc.’s marketing exclusivity rights, this drug product is not labeled with that pediatric information.
14.5 Chronic Musculoskeletal Pain in Adults
Duloxetine is indicated for the management of chronic musculoskeletal pain. This has been established in trials in patients with chronic low
Duloxetine is indicated for the management of chronic musculoskeletal pain. This has been established in trials in patients with chronic low back pain and chronic pain due to osteoarthritis.
Studies in Chronic Low Back Pain
The efficacy of duloxetine delayed-release capsules in adults with chronic low back pain (Study CLBP) was assessed in two double-blind, placebo- controlled, randomized clinical trials of 13 weeks duration (Study CLBP-1 and Study CLBP-2), and one of 12 weeks duration (Study CLBP-3). Study CLBP-1 and Study CLBP-3 demonstrated efficacy of duloxetine delayed-release capsules in the treatment of chronic low back pain. Patients in all trials had no signs of radiculopathy or spinal stenosis.
Study CLBP-1: Two hundred thirty-six adult patients (N = 115 on duloxetine delayed-release capsules, N = 121 on placebo) enrolled and 182 (77%) completed 13-week treatment phase. After 7 weeks of treatment, duloxetine delayed-release capsules patients with less than 30% reduction in average daily pain and who were able to tolerate duloxetine delayed-release capsules 60 mg once daily had their dose of duloxetine delayed-release capsules, in a double-blinded fashion, increased to 120 mg once daily for the remainder of the study. Patients had a mean baseline pain rating of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 13 weeks of treatment, patients taking duloxetine delayed-release capsules 60 to 120 mg daily had a significantly greater pain reduction compared to placebo. Randomization was stratified by the patients’ baseline NSAIDs-use status. Subgroup analyses did not indicate that there were differences in treatment outcomes as a function of NSAIDs use.
Study CLBP-2: Four hundred and four patients were randomized to receive fixed doses of duloxetine delayed-release capsules daily or a matching placebo (N = 59 on duloxetine delayed-release capsules 20 mg, N = 116 on duloxetine delayed-release capsules 60 mg, N = 112 on duloxetine delayed-release capsules 120 mg, N = 117 on placebo) and 267 (66%) completed the entire 13-week study. After 13 weeks of treatment, none of the three duloxetine delayed-release capsules doses showed a statistically significant difference in pain reduction compared to placebo.
Study CLBP-3: Four hundred and one patients were randomized to receive fixed doses of duloxetine delayed-release capsules 60 mg daily or placebo (N = 198 on duloxetine delayed-release capsules, N = 203 on placebo), and 303 (76%) completed the study. Patients had a mean baseline pain rating of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 12 weeks of treatment, patients taking duloxetine delayed-release capsules 60 mg daily had significantly greater pain reduction compared to placebo.
For various degrees of improvement in pain from baseline to study endpoint, Figures 7 and 8 show the fraction of patients in Studies CLBP-1 and CLBP-3 achieving that degree of improvement. The figures are cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned the value of 0% improvement.
Figure 7: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity – Study CLBP-1
Figure 8: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity – Study CLBP-3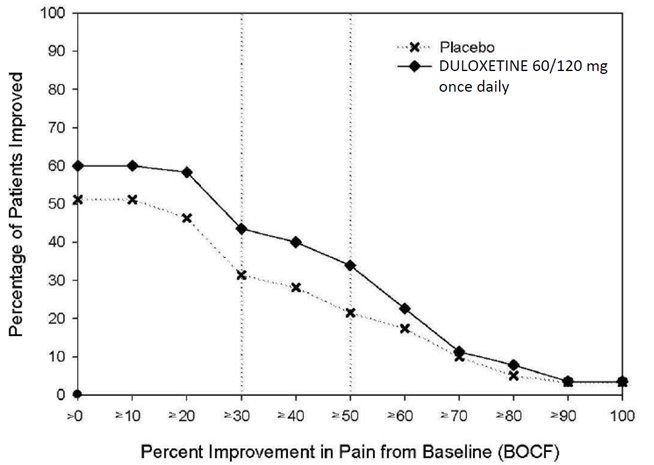 Trials in Chronic Pain Due to Osteoarthritis in Adults
Trials in Chronic Pain Due to Osteoarthritis in Adults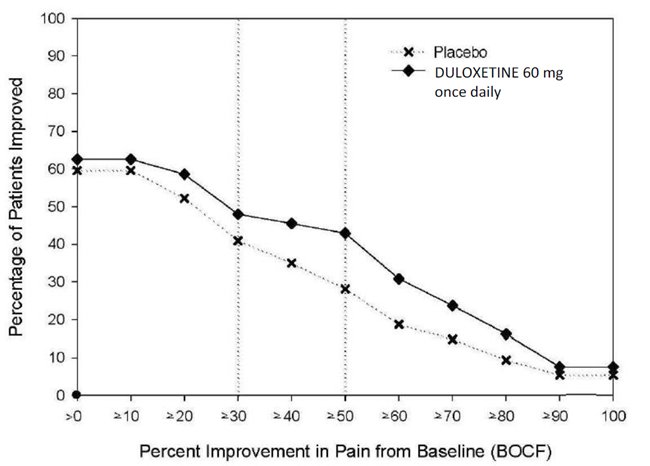
The efficacy of duloxetine delayed-release capsules in chronic pain due to osteoarthritis was assessed in 2 double-blind, placebo- controlled, randomized clinical trials of 13 weeks duration (Study OA-1 and Study OA-2). All patients in both trials fulfilled the ACR clinical and radiographic criteria for classification of idiopathic osteoarthritis of the knee. Randomization was stratified by the patients’ baseline NSAIDs-use status.
Patients assigned to duloxetine delayed-release capsules started treatment in both trials at a dose of 30 mg once daily for one week. After the first week, the dose of duloxetine delayed-release capsules was increased to 60 mg once daily. After 7 weeks of treatment with duloxetine delayed-release capsules 60 mg once daily, in Study OA-1 patients with sub-optimal response to treatment (<30% pain reduction) and tolerated duloxetine delayed-release capsules 60 mg once daily had their dose increased to 120 mg. However, in Study OA-2, all patients, regardless of their response to treatment after 7 weeks, were re-randomized to either continue receiving duloxetine delayed-release capsules 60 mg once daily or have their dose increased to 120 mg once daily for the remainder of the study. Patients in the placebo treatment groups in both trials received a matching placebo for the entire duration of studies. For both studies, efficacy analyses were conducted using 13-week data from the combined duloxetine delayed-release capsules 60 mg and 120 mg once daily treatment groups compared to the placebo group.
Study OA-1: Two hundred fifty-six patients (N = 128 on duloxetine delayed-release capsules, N = 128 on placebo) enrolled and 204 (80%) completed the study. Patients had a mean baseline pain rating of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 13 weeks of treatment, patients taking duloxetine delayed-release capsules had significantly greater pain reduction. Subgroup analyses did not indicate that there were differences in treatment outcomes as a function of NSAIDs use.
Study OA-2: Two hundred thirty-one patients (N = 111 on duloxetine delayed-release capsules, N = 120 on placebo) enrolled and 173 (75%) completed the study. Patients had a mean baseline pain of 6 on a numerical rating scale ranging from 0 (no pain) to 10 (worst possible pain). After 13 weeks of treatment, patients taking duloxetine delayed-release capsules did not show a significantly greater pain reduction.
In Study OA-1, for various degrees of improvement in pain from baseline to study endpoint, Figure 9 shows the fraction of patients achieving that degree of improvement. The figure is cumulative, so that patients whose change from baseline is, for example, 50%, are also included at every level of improvement below 50%. Patients who did not complete the study were assigned the value of 0% improvement.
Figure 9: Percentage of Patients Achieving Various Levels of Pain Relief as Measured by 24-Hour Average Pain Severity – Study OA-1
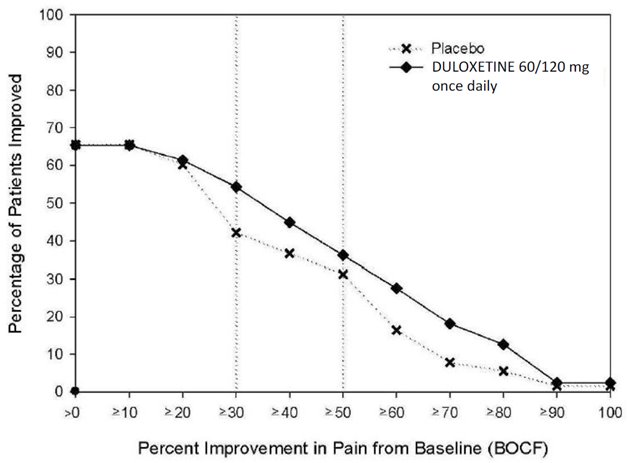
-
16 HOW SUPPLIED/STORAGE AND HANDLING
16.1 How Supplied
DRIZALMA SPRINKLE (duloxetine delayed-release capsules) are available as follows:
Features
Strengths
20 mg a
30 mg a
40 mg a
60 mg a
Body color
Green
White
White
Green
Cap color
Green
Blue
White
Blue
Cap imprint
RG53
RG54
RL85
RG55
Body imprint
RG53
RG54
RL85
RG55
Presentations and NDC Codes
Bottles of 30
47335-616-30
47335-617-30
47335-618-30
47335-619-30
Bottles of 60
47335-616-60
47335-617-60
47335-618-60
47335-619-60
Bottles of 90
47335-616-90
47335-617-90
47335-618-90
47335-619-90
Bottles of 1000
47335-616-10
47335-617-10
47335-618-10
47335-619-10
Each DRIZALMA SPRINKLE (duloxetine delayed-release capsule) contains enteric-coated pellets containing a total of 22.4 mg, 33.6 mg, 44.9 mg or 67.3 mg of duloxetine hydrochloride, USP equivalent to 20 mg, 30 mg, 40 mg or 60 mg of duloxetine, respectively.
-
17 PATIENT COUNSELING INFORMATION
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).Suicidal Thoughts and Behaviors - Advise patients, their families and their caregivers to look for the emergence of suicidal ideation and behavior, especially during treatment and when the dose is adjusted up or down, and instruct them to report such symptoms to their healthcare provider [see Boxed Warning, and Warnings and Precautions (5.1)].
Hepatotoxicity - Inform patients that severe liver problems, sometimes fatal, have been reported in patients treated with duloxetine delayed-release capsules. Instruct patients to talk to their healthcare provider if they develop itching, right upper belly pain, dark urine, or yellow skin/eyes while taking DRIZALMA SPRINKLE which may be signs of liver problems. Instruct patients to talk to their healthcare provider about their alcohol consumption. Use of DRIZALMA SPRINKLE with heavy alcohol intake may be associated with severe liver injury [see Warnings and Precautions (5.2)].
Alcohol - Although duloxetine delayed-release capsules does not increase the impairment of mental and motor skills caused by alcohol, use of DRIZALMA SPRINKLE concomitantly with heavy alcohol intake may be associated with severe liver injury. For this reason, DRIZALMA SPRINKLE should not be prescribed for patients with substantial alcohol use [see Warnings and Precautions (5.2) and Drug Interactions (7.1)].
Orthostatic Hypotension, Falls and Syncope - Advise patients of the risk of orthostatic hypotension, falls and syncope, especially during the period of initial use and subsequent dose escalation, and in association with the use of concomitant drugs that might potentiate the orthostatic effect of DRIZALMA SPRINKLE [see Warnings and Precautions (5.3)].
Serotonin Syndrome - Caution patients about the risk of serotonin syndrome with the concomitant use of DRIZALMA SPRINKLE and other serotonergic agents including triptans, tricyclic antidepressants, opioids, lithium, buspirone, tryptophan, amphetamines, and St. John’s Wort [see Contraindications (4), Warnings and Precautions (5.4), and Drug Interactions (7)].
Advise patients of the signs and symptoms associated with serotonin syndrome that may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular changes (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination), seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea). Caution patients to seek medical care immediately if they experience these symptoms.
Increased Risk of Bleeding - Caution patients about the concomitant use of DRIZALMA SPRINKLE and NSAIDs, aspirin, warfarin, or other drugs that affect coagulation since combined use of psychotropic drugs that interfere with serotonin reuptake and these agents has been associated with an increased risk of bleeding [see Warnings and Precautions (5.5)].
Severe Skin Reactions - Caution patients that DRIZALMA SPRINKLE may cause serious skin reactions. This may need to be treated in a hospital and may be life-threatening. Counsel patients to call their doctor right away or get emergency help if they have skin blisters, peeling rash, sores in their mouth, hives, or any other allergic reactions [see Warnings and Precautions (5.6)].
Discontinuation of Treatment - Instruct patients that discontinuation of DRIZALMA SPRINKLE may be associated with symptoms such as dizziness, headache, nausea, diarrhea, paresthesia, irritability, vomiting, insomnia, anxiety, hyperhidrosis, and fatigue, and should be advised not to alter their dosing regimen, or stop taking DRIZALMA SPRINKLE without consulting their healthcare provider [see Warnings and Precautions (5.7)].
Activation of Mania or Hypomania - Adequately screen patients with depressive symptoms for risk of bipolar disorder (e.g. family history of suicide, bipolar disorder, and depression) prior to initiating treatment with DRIZALMA SPRINKLE. Advise patients to report any signs or symptoms of a manic reaction such as greatly increased energy, severe trouble sleeping, racing thoughts, reckless behavior, talking more or faster than usual, unusually grand ideas, and excessive happiness or irritability [see Warnings and Precautions (5.8)].
Angle-Closure Glaucoma - Advise patients that taking DRIZALMA SPRINKLE can cause mild pupillary dilation, which in susceptible individuals, can lead to an episode of angle-closure glaucoma. Pre-existing glaucoma is almost always open-angle glaucoma because angle-closure glaucoma, when diagnosed, can be treated definitively with iridectomy. Open-angle glaucoma is not a risk factor for angle-closure glaucoma. Patients may wish to be examined to determine whether they are susceptible to angle-closure, and have a prophylactic procedure (e.g., iridectomy), if they are susceptible [see Warnings and Precautions (5.9)].
Seizures - Advise patients to inform their healthcare provider if they have a history of seizure disorder [see Warnings and Precautions (5.10)].
Effects on Blood Pressure - Caution patients that DRIZALMA SPRINKLE may cause an increase in blood pressure [see Warnings and Precautions (5.11)].
Concomitant Medications - Advise patients to inform their healthcare provider if they are taking, or plan to take, any prescription or over-the-counter medications, since there is a potential for interactions [see Dosage and Administration (2.10), Contraindications (4), Warnings and Precautions (5.4, 5.12), and Drug Interactions (7)].
Hyponatremia - Advise patients that hyponatremia has been reported as a result of treatment with SNRIs and SSRIs, including duloxetine delayed-release capsules. Advise patients of the signs and symptoms of hyponatremia [see Warnings and Precautions (5.13)].
Concomitant Illnesses - Advise patients to inform their healthcare provider about all of their medical conditions [see Warnings and Precautions (5.14)].
Urinary Hesitation and Retention - DRIZALMA SPRINKLE are in a class of medicines that may affect urination. Instruct patients to consult with their healthcare provider if they develop any problems with urine flow [see Warnings and Precautions (5.15)].
Sexual Dysfunction - Advise patients that use of DRIZALMA SPRINKLE may cause symptoms of sexual dysfunction in both male and female patients. Inform patients that they should discuss any changes in sexual function and potential management strategies with their healthcare provider [see Warnings and Precautions (516)].
Pregnancy
• Advise pregnant women to notify their healthcare provider if they become pregnant or intend to become pregnant during treatment with DRIZALMA SPRINKLE.
• Advise patients that DRIZALMA SPRINKLE may increase the risk of neonatal complications requiring prolonged hospitalization, respiratory support and tube feeding.
• Advise pregnant women that there is a risk of relapse with discontinuation of antidepressants.
• Advise patients that there is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to duloxetine during pregnancy [see Use in Specific Populations (8.1)].
Lactation- Advise breastfeeding women using DRIZALMA SPRINKLE to monitor infants for sedation, poor feeding and poor weight gain and to seek medical care if they notice these signs [see Use in Specific Populations (8.2)].
Interference with Psychomotor Performance - DRIZALMA SPRINKLE may be associated with sedation and dizziness. Therefore, caution patients about operating hazardous machinery including automobiles, until they are reasonably certain that DRIZALMA SPRINKLE therapy does not affect their ability to engage in such activities.This product, or its use, may be covered by one or more US patents, including US Patent No. 9,839,626 in addition to others including patents pending.
DRIZALMA SPRINKLE is a trademark of Sun Pharmaceutical Industries Limited.
All other trademarks are the property of their respective owners.
Manufactured by:
Sun Pharmaceutical Industries Limited,
Mohali, INDIA
Distributed by:
Sun Pharmaceutical Industries, Inc.
Cranbury, NJ 08512FDA-13
-
Medication Guide
DRIZALMA SPRINKLE™ (dri zal' mah)
(duloxetine delayed-release capsules)
What is the most important information I should know about DRIZALMA SPRINKLE?
DRIZALMA SPRINKLE may cause serious side effects, including:
•Increased risk of suicidal thoughts or actions. DRIZALMA SPRINKLE and other antidepressant medicines may increase suicidal thoughts and actions in some people 24 years of age and younger, especially within the first few months of treatment or when the dose is changed. •Depression and other serious mental illnesses are the most important causes of suicidal thoughts or actions.How can I watch for and try to prevent suicidal thoughts and actions? oPay close attention to any changes in mood, behavior, actions, thoughts, or feelings, especially sudden changes. This is very important when an antidepressant medicine is started or when the dose is changed.oCall your healthcare provider right away to report new or sudden changes in mood, behavior, thoughts, or feelings.oKeep all follow-up visits with your healthcare provider as scheduled. Call your healthcare provider between visits as needed, especially if you have concerns about symptoms.Call your healthcare provider or get emergency help right away if you or your family member have any of the following symptoms, especially if they are new, worse, or worry you:
•attempts to commit suicide •acting on dangerous impulses •acting aggressive, being angry, or violent •thoughts about suicide or dying •new or worse depression •new or worse anxiety •panic attacks •feeling very agitated or restless •new or worse irritability •trouble sleeping •an extreme increase in activity or talking (mania) •other unusual changes in behavior or mood What is DRIZALMA SPRINKLE?
DRIZALMA SPRINKLE is prescription medicine used to treat:
•A certain type of depression called Major Depressive Disorder (MDD) in adults•Generalized Anxiety Disorder (GAD) in adults and children 7 years of age and older•Diabetic Peripheral Neuropathic Pain (DPNP) in adults•Fibromyalgia (FM) in adults•Chronic Musculoskeletal Pain in adultsIt is not known if DRIZALMA SPRINKLE is safe and effective for use to treat GAD in children less than 7 years of age.
It is not known if DRIZALMA SPRINKLE is safe and effective for use to treat MDD, DPNP, and Chronic Musculoskeletal Pain in children.
Do not take DRIZALMA SPRINKLE if you:
•take a Monoamine Oxidase Inhibitor (MAOI)•have stopped taking an MAOI in the last 14 days•are being treated with the antibiotic linezolid or intravenous methylene blue
Ask your healthcare provider or pharmacist if you are not sure if you take an MAOI, including the antibiotic linezolid or intravenous methylene blue.
Do not start taking an MAOI for a least 5 days after you stop treatment with DRIZALMA SPRINKLE.
Before taking DRIZALMA SPRINKLE, tell your healthcare provider about all your medical conditions, including if you:
•have or have a family history of suicide, bipolar disorder, depression, mania or hypomania•have liver or kidney problems•drink alcohol•have or had had bleeding problems•have glaucoma (high pressure in the eye)•have or have had seizures (convulsions)•have high or low blood pressure•have diabetes or high blood sugar•have or have had heart problems or stroke•have low sodium levels in your blood•have problems urinating (hesitation) or emptying your bladder (urinary retention)•are pregnant or plan to become pregnant. DRIZALMA SPRINKLE may harm your unborn baby. Talk to your healthcare provider about the risks to you and your unborn baby if you take DRIZALMA SPRINKLE during pregnancy.•Tell your healthcare provider right away if you become pregnant or think you are pregnant during treatment with DRIZALMA SPRINKLE.•If you become pregnant during treatment with DRIZALMA SPRINKLE, talk to your healthcare provider about registering with the National Pregnancy Registry for Antidepressants. You can register by calling 1-844-405-6185 or by visiting online at https://womensmentalhealth.org/clinical-and-research-programs/pregnancyregistry/antidepressants/•are breastfeeding or plan to breastfeed. DRIZALMA SPRINKLE passes into your breast milk and may harm your baby. Talk to your healthcare provider about the best way to feed your baby during treatment with DRIZALMA SPRINKLE.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
DRIZALMA SPRINKLE and other medicines may affect each other causing possible serious side effects. DRIZALMA SPRINKLE may affect the way other medicines work and other medicines may affect the way DRIZALMA SPRINKLE works.
What should I tell my healthcare provider before taking DRIZALMA SPRINKLE? Ask if you are not sure:
• medicines to treat migraine headaches known as triptans
• tricyclic antidepressants
• lithium
• tramadol, fentanyl, meperidine, methadone, or other opioids
• tryptophan
• buspirone
• amphetamines
• St. John’s Wort
• other medicines containing desvenlafaxine or venlafaxine
• medicines that can affect blood clotting such as aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), warfarinAsk your healthcare provider if you are not sure if you are taking any of these medicines. Your healthcare provider can tell you if it is safe to take DRIZALMA SPRINKLE with your other medicines.
Do not start or stop any other medicines during treatment with TREATMENT without talking to your healthcare provider first. Stopping DRIZALMA SPRINKLE suddenly may cause you to have serious side effects. See, “What are the possible side effects of DRIZALMA SPRINKLE?”
Know the medicines you take. Keep a list of them to show to your healthcare provider and pharmacist when you get a new medicine.
How should I take DRIZALMA SPRINKLE?
•Take DRIZALMA SPRINKLE exactly as your healthcare provider tells you to take it. Do not change your dose or stop taking DRIZALMA SPRINKLE without talking to your healthcare provider.•Your healthcare provider may need to change the dose of DRIZALMA SPRINKLE until it is the right dose for you.•Take DRIZALMA SPRINKLE with or without food.•Swallow DRIZALMA SPRINKLE whole. Do not chew or crush DRIZALMA SPRINKLE.•If you have trouble swallowing DRIZALMA SPRINKLE whole, you can open the capsule and take the contents with applesauce. See the Instructions for Use at the end of this Medication Guide for instructions on how to take DRIZALMA SPRINKLE with applesauce.•See the Instructions for Use at the end of this Medication Guide for instructions on how to mix and give DRIZALMA SPRINKLE through a nasogastric (NG) tube.•If you miss a dose of DRIZALMA SPRINKLE, take the missed dose as soon as you remember. If it is almost time for the next dose, skip the missed dose and take your next dose at the regular time. Do nottake 2 doses of DRIZALMA SPRINKLE at the same time.
If you take too much DRIZALMA SPRINKLE, call your healthcare provider or poison control center at 1800-222-1222 right away, or go to the nearest hospital emergency room.
What are the possible side effects of DRIZALMA SPRINKLE?
DRIZALMA SPRINKLE may cause serious side effects, including:
•See, “What is the most important information I should know about DRIZALMA SPRINKLE?” •Liver problems. DRIZALMA SPRINKLE may cause severe liver problems that may cause death. Tell your healthcare provider right away if you develop any of the following symptoms of severe liver problems:•Decreased in blood pressure (orthostatic hypotension). You may feel lightheaded or faint when you rise too quickly from a sitting or lying position, especially when you start or restart treatment or when the dose is changed.•Falls and fainting. DRIZALMA SPRINKLE may cause you to feel sleepy or dizzy, may cause a decrease in your blood pressure when you rise to quickly from a sitting or lying position, and can slow your thinking and motor skills which may lead to falls that have caused fractures or other serious injuries.•Serotonin syndrome. A potentially life-threatening problem called serotonin syndrome can happen when you take DRIZALMA SPRINKLE with certain other medicines. See, “Who should not take DRIZALMA SPRINKLE?” Call your healthcare provider or go to the nearest hospital emergency room right away if you have any of the following signs and symptoms of serotonin syndrome:•Abnormal bleeding. Taking DRIZALMA SPRINKLE with aspirin, non-steroidal anti-inflammatory drugs (NSAIDs), or blood thinners may add to this risk. Tell your healthcare provider right away about any unusual bleeding or bruising.•Severe skin reactions. DRIZALMA SPRINKLE may cause serious skin reactions that may need to be treated in a hospital and may be life-threating. Stop taking DRIZALMA SPRINKLE and call your healthcare provider or get emergency help right away if you develop skin blisters, peeling rash, sores in the mouth, hives or any other allergic reactions during treatment with DRIZALMA SPRINKLE.•Discontinuation syndrome. Suddenly stopping DRIZALMA SPRINKLE when you take higher doses may cause you to have serious side effects. Your healthcare provider may want to decrease your dose slowly. Symptoms may include:•Manic episodes. Manic episodes may happen in people with bipolar disorder who take DRIZALMA SPRINKLE. Symptoms may include:•Eye problems (angle-closure glaucoma). DRIZALMA SPRINKLE may cause a type of eye problem called angle-closure glaucoma in people with certain other eye conditions. You may want to undergo an eye examination to see if you are at risk and receive preventative treatment if you are. Call your healthcare provider if you have eye pain, changes in your vision, or swelling or redness in or around the eye. Seizures (convulsions). •Increases in blood pressure. Your healthcare provider should check your blood pressure regularly during treatment with DRIZALMA SPRINKLE. If you have high blood pressure, it should be controlled before you start treatment with DRIZALMA SPRINKLE.•Low sodium levels in your blood (hyponatremia). Low sodium levels can happen during treatment with DRIZALMA SPRINKLE. Low sodium levels in your blood may be serious and may cause death. Elderly people may be at greater risk for this. Signs and Symptoms of low sodium levels in your blood may include:oheadacheodifficulty concentratingomemory changesoconfusionoweakness and unsteadiness on your feet which can lead to fallsIn severe or more sudden cases, signs and symptoms include:ohallucinations (seeing or hearing things that are not real)ofaintingoseizuresocomaorespiratory arrestodeath•Problems with urination. DRIZALMA SPRINKLE may cause you to have problems with urination including decreased urine flow and being unable to pass any urine. Tell your healthcare provider if you develop any problems with urine flow during treatment with DRIZALMA SPRINKLE.•Sexual problems (dysfunction).Taking serotonin and norepinephrine reuptake inhibitors (SNRIs), including DRIZALMA SPRINKLE, may cause sexual problems.Symptoms in males may include:oDelayed ejaculation or inability to have an ejaculationoDecreased sex driveoProblems getting or keeping an erectionSymptoms in females may include:oDecreased sex driveoDelayed orgasm or inability to have an orgasmTalk to your healthcare provider if you develop any changes in your sexual function or if you have any questions or concerns about sexual problems during treatment with DRIZALMA SPRINKLE. There may be treatments your healthcare provider can suggest. Your healthcare provider may tell you to stop taking DRIZALMA SPRINKLE if you develop serious side effects during treatment with DRIZALMA SPRINKLE.
The most common side effects of DRIZALMA SPRINKLE in adults include:
The most common side effects of DRIZALMA SPRINKLE in children include:
Height and weight changes in children and adolescents may happen during treatment with DRIZALMA SPRINKLE. Your healthcare provider should check your child’s or adolescent’s height and weight during treatment with DRIZALMA SPRINKLE.
These are not all the possible side effects of DRIZALMA SPRINKLE.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
How should I store DRIZALMA SPRINKLE?
•Store DRIZALMA SPRINKLE at room temperature between 68°F to 77°F (20°C to 25°C).•Store DRIZALMA SPRINKLE in a tightly closed container.Keep DRIZALMA SPRINKLE and all medicines out of the reach of children.
General information about the safe and effective use of DRIZALMA SPRINKLE.
Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. Do not use DRIZALMA SPRINKLE for a condition for which it was not prescribed. Do not give DRIZALMA SPRINKLE to other people, even if they have the same symptoms that you have. It may harm them. You may ask your healthcare provider or pharmacist for information about DRIZALMA SPRINKLE that is written for healthcare professionals.
What are the ingredients in DRIZALMA SPRINKLE?
Active ingredient: duloxetine hydrochloride
Inactive ingredients: ascorbic acid, hypromellose, hypromellose phthalate, polyethylene glycol, starch, sucrose, sugar spheres, talc, titanium dioxide, and triethyl citrate.
The capsule shell ingredients for 20 mg strength are D&C Yellow 10, FD &C Blue 1, FD &C Red 40, gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 30 mg strength are FD &C Blue 1, FD &C Red 40 and FD &C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 40 mg strength are gelatin, sodium lauryl sulfate and titanium dioxide. The capsule shell ingredients for 60 mg strength are D&C Yellow 10 (present in body), FD &C Blue 1, FD &C Red 40, FD &C Red 3 (present in cap), gelatin, sodium lauryl sulfate and titanium dioxide.The imprinting ink for 20 mg, 30 mg, 40 mg, and 60 mg strength capsules was made of ammonia solution, black iron oxide, butyl alcohol, dehydrated alcohol, isopropyl alcohol, potassium hydroxide, propylene glycol and shellac.Manufactured by:Sun Pharmaceutical Industries Limited,Mohali, INDIADistributed by:Sun Pharmaceutical Industries, Inc.Cranbury, NJ 08512FDA-05For more information call Sun Pharmaceutical Industries, Inc. at 1-800-818-4555.
This Medication Guide has been approved by the U.S. Food and Drug Administration. Revised: August/2023
-
Instructions for Use
DRIZALMA SPRINKLE (dri zal' mah)
(duloxetine delayed-release capsules)
Taking DRIZALMA SPRINKLE with applesauce:
1.Carefully open the DRIZALMA SPRINKLE capsule.2.Sprinkle the pellets from the capsules on 1 tablespoonful of applesauce.3.Swallow the applesauce and pellets mixture right away. Do not chew the pellets.4.Do not save the applesauce and pellets mixture for later use. Throw away any remaining applesauce and pellets mixture.
Giving DRIZALMA SPRINKLE through a nasogastric tube (NG tube) 12 French or larger as prescribed by your healthcare provider:
For people who have a NG tube in place, DRIZALMA SPRINKLE may be given as follows:
•Remove the plunger from a 60 mL catheter tipped syringe.•Carefully open the DRIZALMA SPRINKLE capsule and empty the pellets into the catheter tipped syringe barrel.•Add 50 mL of water to the pellets that are inside of the catheter tipped syringe barrel. Do not use other liquids.•Replace the plunger and gently shake the syringe well for approximately 10 seconds.•Insert the catheter tipped syringe to a NG tube (≥12 French).•Give the mixture right away through the NG tube into the stomach. Do not save the mixture for later use.•After giving the mixture, the NG tube should be flushed with 15 mL of additional water.
How should I store DRIZALMA SPRINKLE?
•Store DRIZALMA SPRINKLE at room temperature between 68°F to 77°F (20°C to 25°C).•Store DRIZALMA SPRINKLE in a tightly closed container. Keep DRIZALMA SPRINKLE and all medicines out of the reach of children.Manufactured by:Sun Pharmaceutical Industries Limited,Mohali, INDIADistributed by:Sun Pharmaceutical Industries, Inc.Cranbury, NJ 08512FDA-02
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Issued: July/2021
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
- PACKAGE/LABEL PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
DRIZALMA SPRINKLE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47335-616 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 20 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) STARCH, CORN (UNII: O8232NY3SJ) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) SUCROSE (UNII: C151H8M554) ASCORBIC ACID (UNII: PQ6CK8PD0R) AMMONIA (UNII: 5138Q19F1X) Product Characteristics Color GREEN (green body) , GREEN (green cap) Score no score Shape CAPSULE Size 14mm Flavor Imprint Code RG;53 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47335-616-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 2 NDC:47335-616-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 3 NDC:47335-616-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 4 NDC:47335-616-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212516 06/10/2024 DRIZALMA SPRINKLE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47335-617 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 30 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ASCORBIC ACID (UNII: PQ6CK8PD0R) Product Characteristics Color WHITE (white body) , BLUE (blue cap) Score no score Shape CAPSULE Size 16mm Flavor Imprint Code RG;54 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47335-617-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 2 NDC:47335-617-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 3 NDC:47335-617-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 4 NDC:47335-617-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212516 06/10/2024 DRIZALMA SPRINKLE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47335-618 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 40 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) SUCROSE (UNII: C151H8M554) STARCH, CORN (UNII: O8232NY3SJ) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ASCORBIC ACID (UNII: PQ6CK8PD0R) Product Characteristics Color WHITE (white body) , WHITE (white cap) Score no score Shape CAPSULE Size 18mm Flavor Imprint Code RL;85 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47335-618-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 2 NDC:47335-618-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 3 NDC:47335-618-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 4 NDC:47335-618-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212516 06/10/2024 DRIZALMA SPRINKLE
duloxetine capsule, delayed releaseProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:47335-619 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DULOXETINE HYDROCHLORIDE (UNII: 9044SC542W) (DULOXETINE - UNII:O5TNM5N07U) DULOXETINE 60 mg Inactive Ingredients Ingredient Name Strength TALC (UNII: 7SEV7J4R1U) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) D&C YELLOW NO. 10 (UNII: 35SW5USQ3G) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GELATIN, UNSPECIFIED (UNII: 2G86QN327L) SODIUM LAURYL SULFATE (UNII: 368GB5141J) FERROSOFERRIC OXIDE (UNII: XM0M87F357) SHELLAC (UNII: 46N107B71O) AMMONIA (UNII: 5138Q19F1X) FD&C RED NO. 3 (UNII: PN2ZH5LOQY) TRIETHYL CITRATE (UNII: 8Z96QXD6UM) STARCH, CORN (UNII: O8232NY3SJ) SUCROSE (UNII: C151H8M554) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) HYPROMELLOSE PHTHALATE (24% PHTHALATE, 55 CST) (UNII: 87Y6436BKR) HYPROMELLOSE, UNSPECIFIED (UNII: 3NXW29V3WO) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) ASCORBIC ACID (UNII: PQ6CK8PD0R) Product Characteristics Color GREEN (green body) , BLUE (blue cap) Score no score Shape CAPSULE Size 20mm Flavor Imprint Code RG;55 Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:47335-619-30 30 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 2 NDC:47335-619-90 90 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 3 NDC:47335-619-10 1000 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 4 NDC:47335-619-60 60 in 1 BOTTLE; Type 0: Not a Combination Product 06/10/2024 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date NDA NDA212516 06/10/2024 Labeler - SUN PHARMACEUTICAL INDUSTRIES, INC. (146974886) Registrant - SUN PHARMACEUTICAL INDUSTRIES, INC. (146974886) Establishment Name Address ID/FEI Business Operations Sun Pharmaceutical Industries Limited 650456002 MANUFACTURE(47335-616, 47335-617, 47335-618, 47335-619)








