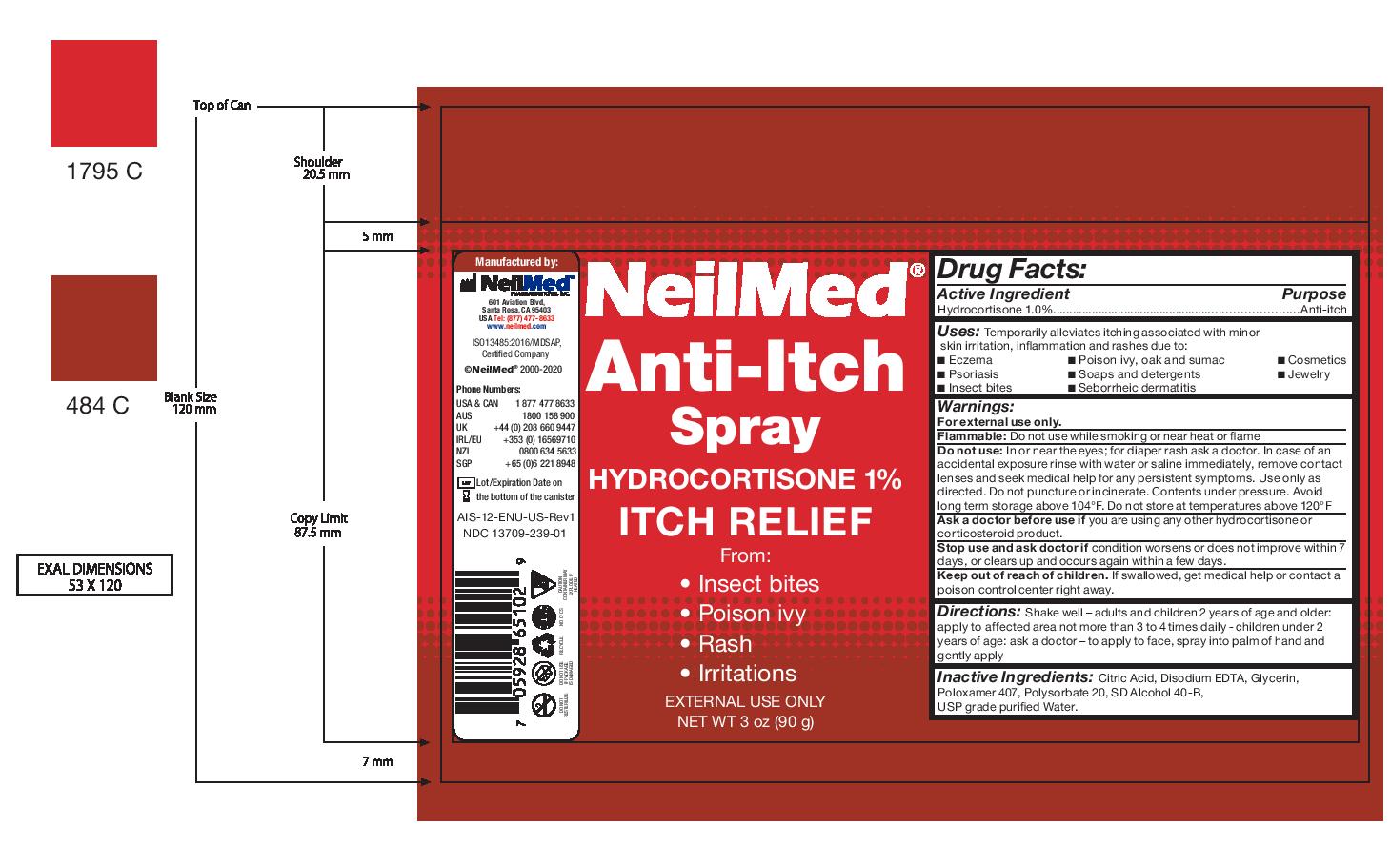
Label: NEILMED ANTI-ITCH- anti-itch spray spray
- NDC Code(s): 13709-239-01
- Packager: NeilMed Pharmaceuticals Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated January 11, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Directions:
-
Warnings:
For external use only.
Flammable: Do not use while smoking or near heat or flame
Do not use: In or near the eyes; for diaper rash ask a doctor. In case of an accidental exposure rinse with water or saline immediately, remove contact lenses and seek medical help for any persistent symptoms. Use only as directed. Do not puncture or incinerate. Contents under pressure. Avoid long term storage above 104°F. Do not store at temperatures above 120°F
Ask a doctor before use if you are using any other hydrocortisone or corticosteroid product.
Stop use and ask doctor if condition worsens or does not improve within 7 days, or clears up and occurs again within a few days.
Keep out of reach of children. If swallowed, get medical help or contact a poison control center right away. - Inactive Ingredients:
- Uses:
- Keep out of reach of children.
- Drug Facts: Purpose
- Drug Facts:
- NeilMed Anti Itch Spray
-
INGREDIENTS AND APPEARANCE
NEILMED ANTI-ITCH
anti-itch spray sprayProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:13709-239 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYDROCORTISONE (UNII: WI4X0X7BPJ) (HYDROCORTISONE - UNII:WI4X0X7BPJ) HYDROCORTISONE 1 g in 1 g Inactive Ingredients Ingredient Name Strength EDETATE DISODIUM (UNII: 7FLD91C86K) ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) ALCOHOL (UNII: 3K9958V90M) POLYSORBATE 20 (UNII: 7T1F30V5YH) GLYCERIN (UNII: PDC6A3C0OX) POLOXAMER 407 (UNII: TUF2IVW3M2) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:13709-239-01 90 g in 1 CAN; Type 0: Not a Combination Product 11/11/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M017 11/11/2020 Labeler - NeilMed Pharmaceuticals Inc. (799295915) Establishment Name Address ID/FEI Business Operations NeilMed Pharmaceuticals, Inc. 799295915 manufacture(13709-239)