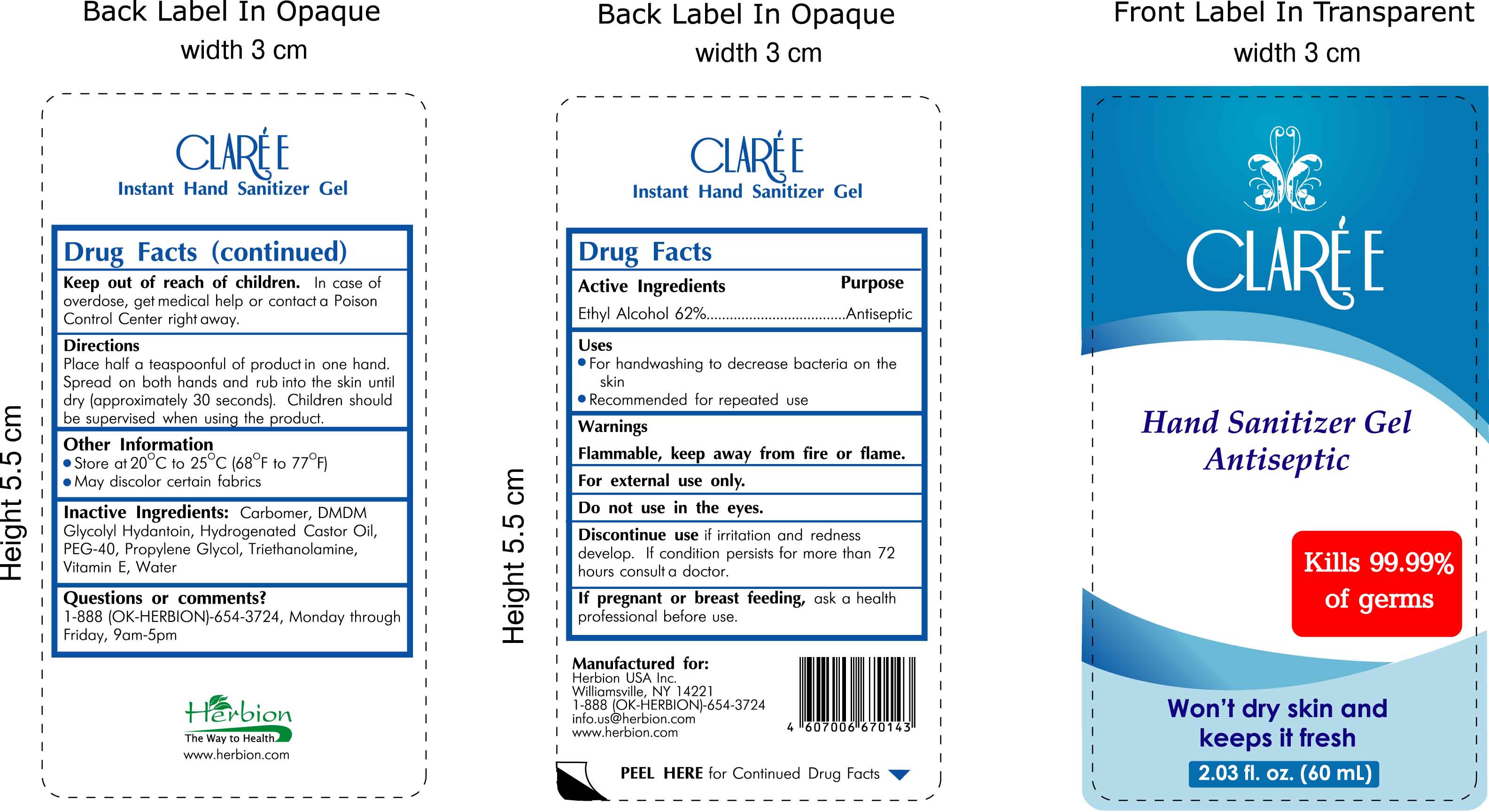
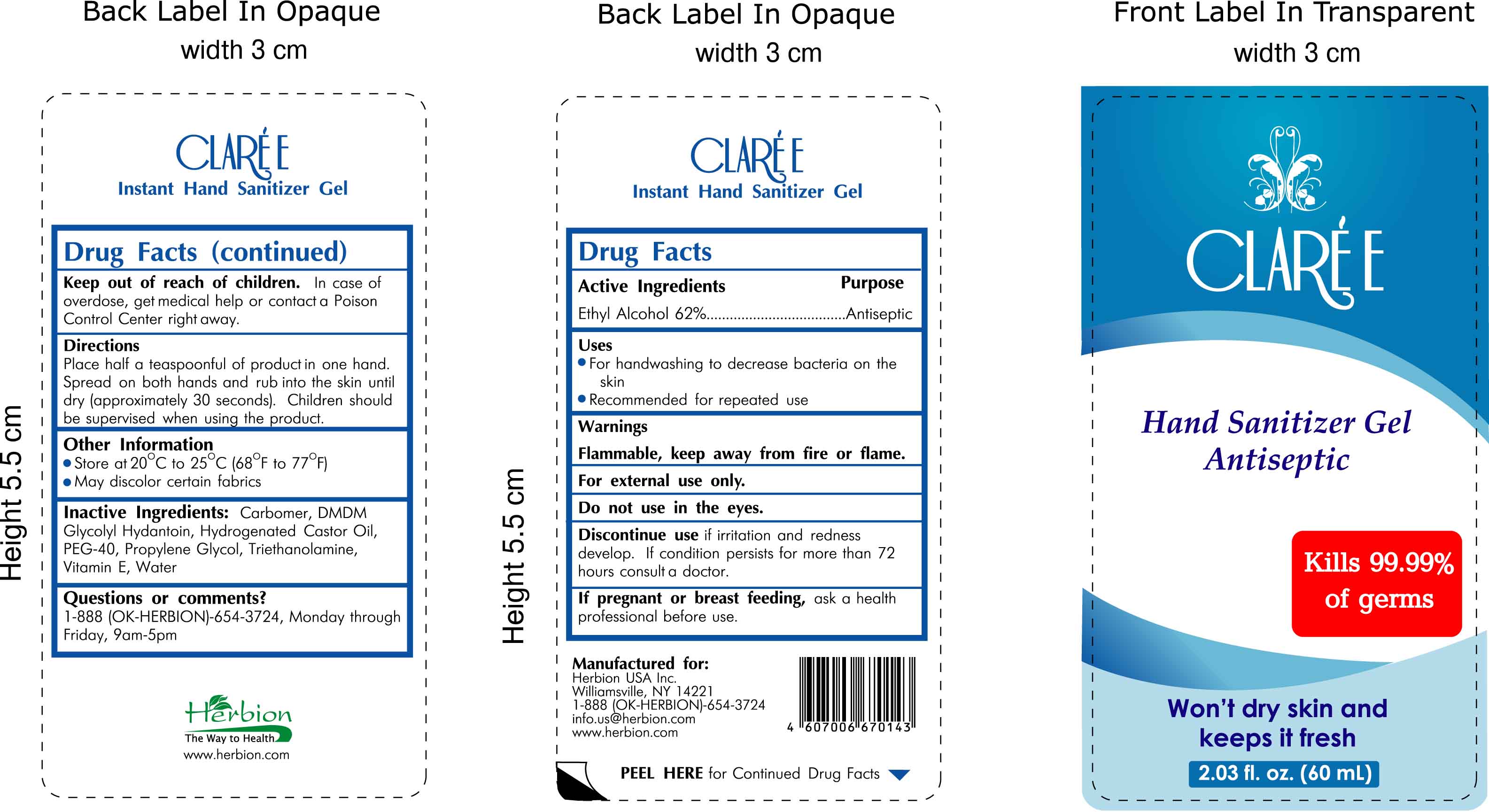
Label: CLAREE- hand sanitizer gel antiseptic gel
-
Contains inactivated NDC Code(s)
NDC Code(s): 44237-400-60 - Packager: Herbion Pakistan (Pvt.) Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 29, 2010
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- INDICATIONS & USAGE
- DOSAGE & ADMINISTRATION
-
WARNINGS AND PRECAUTIONS
Warnings:
Flammable, keep away from fire or flame.
For external use only.
Do not use in the eyes.
Discontinue use if irritation and redness develop. If condition persists for more than 72 hours consult a doctor.
If pregnant or breast feeding, ask a health professional before use.
Keep out of reach of children. In case of overdose, get medical help or contact a Poison Control Center right away.
Other Information:
- Store at 20 (degrees C) to 25 (degrees C) [68 degrees F to 77 degrees F]
- May discolor certain fabrics
- INACTIVE INGREDIENT
- ACTIVE INGREDIENT
- DESCRIPTION
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
CLAREE
hand sanitizer gel antiseptic gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:44237-400 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 37 mL in 60 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:44237-400-60 60 mL in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph final part333 04/01/2010 Labeler - Herbion Pakistan (Pvt.) Ltd. (645482936) Registrant - Herbion Pakistan (Pvt.) Ltd. (645482936) Establishment Name Address ID/FEI Business Operations Herbion Pakistan (Pvt.) Ltd. 645482936 manufacture