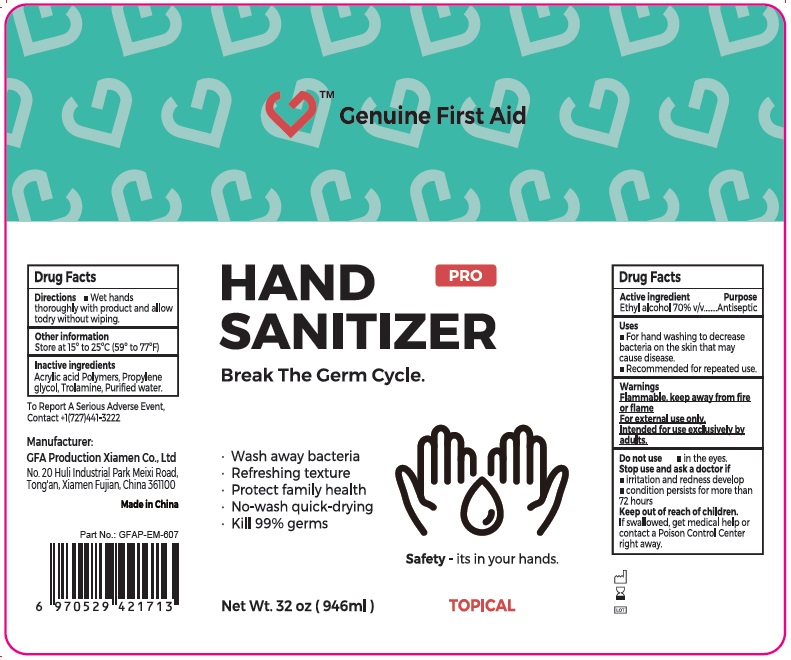
Label: GENUINE FIRST AID HAND SANITIZER ETHYL ALCOHOL- alcohol gel
- NDC Code(s): 50814-063-01, 50814-063-02
- Packager: GFA Production (Xiamen) Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated October 25, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Drug Facts
- Active ingredient
- Uses
- Warnings
- Directions
- Other information
- Inactive ingredients
- Package Labeling:473ml
- Package Labeling:946ml
-
INGREDIENTS AND APPEARANCE
GENUINE FIRST AID HAND SANITIZER ETHYL ALCOHOL
alcohol gelProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:50814-063 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 0.7 mL in 1 mL Inactive Ingredients Ingredient Name Strength PROPYLENE GLYCOL (UNII: 6DC9Q167V3) TROLAMINE (UNII: 9O3K93S3TK) WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50814-063-01 473 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2020 2 NDC:50814-063-02 946 mL in 1 BOTTLE; Type 0: Not a Combination Product 08/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug 505G(a)(3) 08/01/2020 Labeler - GFA Production (Xiamen) Co., Ltd. (421256261)