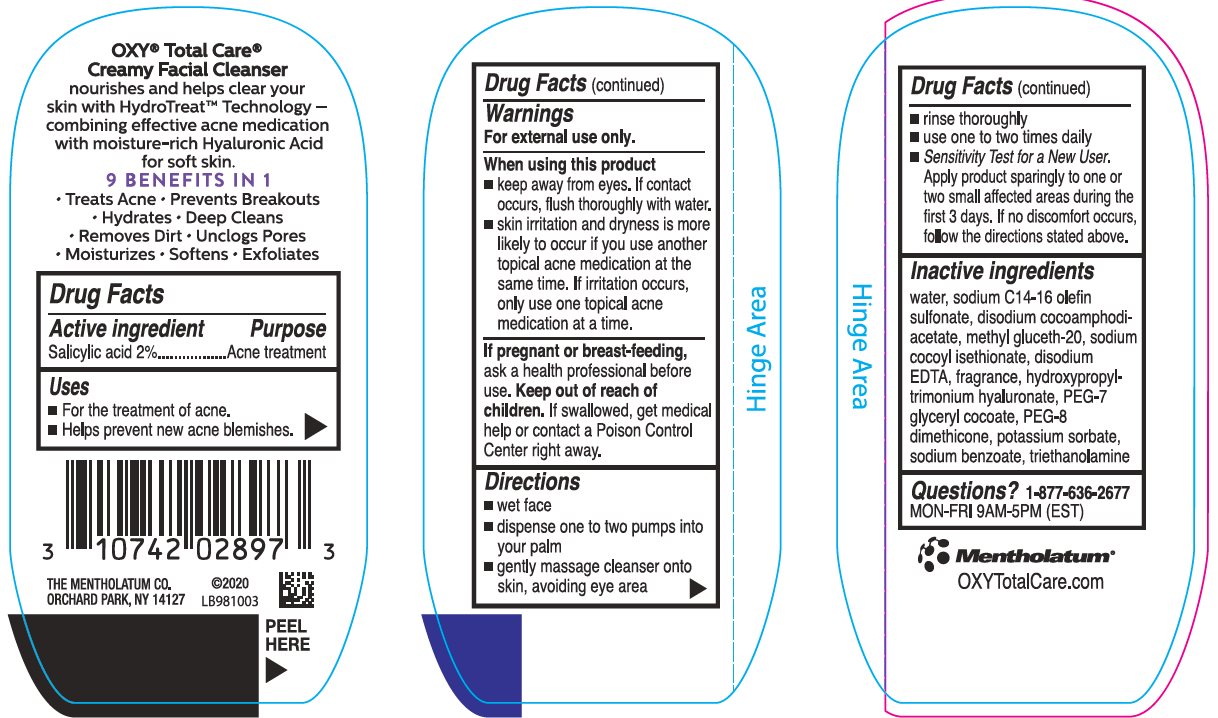
Label: OXY TOTAL CARE CREAMY FACIAL CLEANSER- salicylic acid cream
- NDC Code(s): 10742-1316-1
- Packager: The Mentholatum Company
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 13, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Keep Out of Reach of Children
- Uses
-
Warnings
For external use only.
When using this product
- keep away from eyes. If contact occurs, flush thoroughly with water.
- skin irritation and dryness is more likely to occur if you use another topical acne medication at the same time. If irritation occurs, use only one topical acne medication at a time.
If pregnant or breast-feeding, ask a health professional before use.
-
Directions
- wet face
- dispense one to two pumps into your palm
- gently massage cleanser onto skin, avoiding eye area
- rinse thoroughly
- use one to two times daily
- Sensitivity Test for a New User. Apply product sparingly to one or two small affected areas during the first 3 days. If no discomfort occurs, follow the directions stated above.
-
Inactive Ingredients
water, sodium C14-16 olefin sulfonate, disodium cocoamphodiacetate, methyl gluceth-20, sodium cocoyl isethionate, disodium EDTA, fragrance, hydroxypropyltrimonium hyaluronate, PEG-7 glyceryl cocoate, PEG-8 dimethicone, potassium sorbate, sodium benzoate, triethanolamone
Questions?1-877-636-2677
MON-FRI 9AM-5PM (EST)
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
OXY TOTAL CARE CREAMY FACIAL CLEANSER
salicylic acid creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:10742-1316 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength SALICYLIC ACID (UNII: O414PZ4LPZ) (SALICYLIC ACID - UNII:O414PZ4LPZ) SALICYLIC ACID 20 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) SODIUM C14-16 OLEFIN SULFONATE (UNII: O9W3D3YF5U) DISODIUM COCOAMPHODIACETATE (UNII: 18L9G3U51M) METHYL GLUCETH-20 (UNII: J3QD0LD11P) SODIUM COCOYL ISETHIONATE (UNII: 518XTE8493) EDETATE DISODIUM (UNII: 7FLD91C86K) HYALURONATE SODIUM (UNII: YSE9PPT4TH) PEG-7 GLYCERYL COCOATE (UNII: VNX7251543) PEG-8 DIMETHICONE (UNII: GIA7T764OD) POTASSIUM SORBATE (UNII: 1VPU26JZZ4) SODIUM BENZOATE (UNII: OJ245FE5EU) TROLAMINE (UNII: 9O3K93S3TK) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:10742-1316-1 148 mL in 1 TUBE; Type 0: Not a Combination Product 09/30/2019 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M006 09/30/2019 Labeler - The Mentholatum Company (002105757) Registrant - The Mentholatum Company (002105757) Establishment Name Address ID/FEI Business Operations The Mentholatum Company 002105757 manufacture(10742-1316)