Label: EMULSIVIT E AD- d-alpha tocopherol vitamin a vitamin d3 injection
- NDC Code(s): 50989-344-15
- Packager: Vedco, Inc.
- Category: OTC ANIMAL DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated December 11, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
-
INDICATIONS & USAGE
EmulsiVit E/A&D
Injectable Tocopherol/AD
Indications: EmulsiVitE/A&D Injectable d-alpha tocopherol with AD is a clear, sterile, non-aqueous solution of vitamin A,vitamin D3,and vitamin E. The product is intended as a supplemental source of vitamins A, D and E.
Natural vitamin A (carotenes), and tocopherols can be destroyed in feedstuffs through processing, ensiling and storage. Due to these losses, reduced intakes of fat-soluble vitamins can occur in animals maintained in continual confinement compared to animals allowed to graze in lush pasture. Intramuscular or subcutaneous injections offer an efficient and rapid method to increase vitamin A, vitamin D and Vitamin E status of animals.
-
Each mL contains:
Active Ingredients:
vitamin E (as d-alpha-tocopherol,
a natural source of vitamin E).....................................300 IU
Vitamin A Propionate...........................................100,000 IU
Vitamin D3...........................................................10,000 IU
Inactive Ingredients: 2% benzyl alcohol (preservative)
compounder in a water emulsifiable base.
-
Caution:
Anaphylactoid or allergic reactions, which have resulted in deaths, have been reported in individual animals as well as entire herds. Should such reactions or hypersensitivity occur, treat immediately with injection of epinephrine and/or antihistamines. Do not exceed recomended dosage. Administration of this product to well-nourished animals may cause hypervitaminosis D, which may result in hypercalcemia and hyperphosphatemia.
- Warning:
- STORAGE AND HANDLING
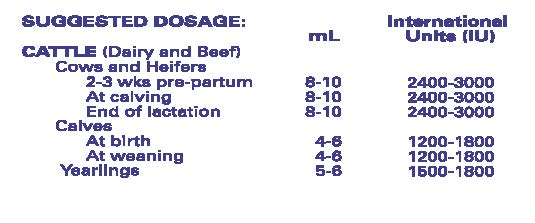
- ADMINISTRATION AND DOSAGE:
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
EMULSIVIT E AD
d-alpha tocopherol vitamin a vitamin d3 injectionProduct Information Product Type OTC ANIMAL DRUG Item Code (Source) NDC:50989-344 Route of Administration INTRAMUSCULAR, SUBCUTANEOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength .ALPHA.-TOCOPHEROL, D- (UNII: N9PR3490H9) (.ALPHA.-TOCOPHEROL, D- - UNII:N9PR3490H9) .ALPHA.-TOCOPHEROL, D- 201 mg in 1 mL VITAMIN A (UNII: 81G40H8B0T) (VITAMIN A - UNII:81G40H8B0T) VITAMIN A 3.6 mg in 1 mL VITAMIN D (UNII: 9VU1KI44GP) (CHOLECALCIFEROL - UNII:1C6V77QF41) VITAMIN D 2.5 mg in 1 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:50989-344-15 250 mL in 1 VIAL Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 01/10/2010 Labeler - Vedco, Inc. (021634266)