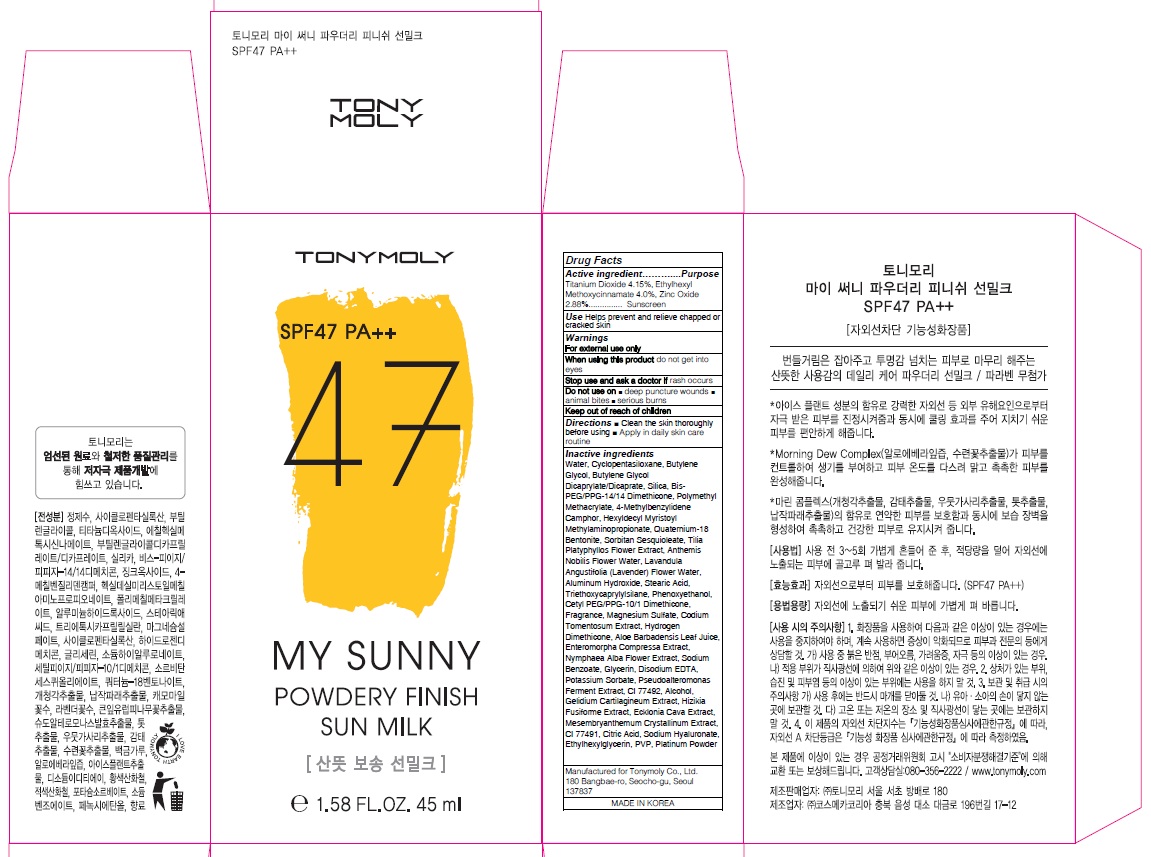
Label: MY SUNNY POWDE RY FINISH SUN MILK- titanium dioxide, octinoxate, zinc oxide cream
-
Contains inactivated NDC Code(s)
NDC Code(s): 59078-309-01, 59078-309-02 - Packager: TONYMOLY CO.,LTD
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated June 14, 2016
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
-
INACTIVE INGREDIENT
Inactive Ingredient: Water, Cyclopentasiloxane, Butylene Glycol, Butylene Glycol Dicaprylate/Dicaprate, Silica, Bis-PEG/PPG-14/14 Dimethicone, Polymethyl Methacrylate, 4-Methylbenzylidene Camphor, Hexyldecyl Myristoyl Methylaminopropionate, Quaternium-18 Bentonite, Sorbitan Sesquioleate, Tilia Platyphyllos Flower Extract, Anthemis Nobilis Flower Water, Lavandula Angustifolia (Lavender) Flower Water, Aluminum Hydroxide, Stearic Acid, Triethoxycaprylylsilane, Phenoxyethanol, Cetyl PEG/PPG-10/1 Dimethicone, Fragrance, Magnesium Sulfate, Codium Tomentosum Extract, Hydrogen Dimethicone, Aloe Barbadensis Leaf Juice, Enteromorpha Compressa Extract, Nymphaea Alba Flower Extract, Sodium Benzoate, Glycerin, Disodium EDTA, Potassium Sorbate, Pseudoalteromonas Ferment Extract, CI 77492, Alcohol, Gelidium Cartilagineum Extract, Hizikia Fusiforme Extract, Ecklonia Cava Extract, Mesembryanthemum Crystallinum Extract, CI 77491, Citric Acid, Sodium Hyaluronate, Ethylhexylglycerin, PVP, Platinum Powder
- PURPOSE
- WARNINGS
- KEEP OUT OF REACH OF CHILDREN
- DESCRIPTION
- PACKAGE LABEL.PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
MY SUNNY POWDE RY FINISH SUN MILK
titanium dioxide, octinoxate, zinc oxide creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59078-309 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength Titanium Dioxide (UNII: 15FIX9V2JP) (TITANIUM DIOXIDE - UNII:15FIX9V2JP) Titanium Dioxide 1.86 g in 45 mL Octinoxate (UNII: 4Y5P7MUD51) (OCTINOXATE - UNII:4Y5P7MUD51) Octinoxate 1.80 g in 45 mL Zinc Oxide (UNII: SOI2LOH54Z) (ZINC CATION - UNII:13S1S8SF37) ZINC CATION 1.29 g in 45 mL Inactive Ingredients Ingredient Name Strength Water (UNII: 059QF0KO0R) Butylene Glycol (UNII: 3XUS85K0RA) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59078-309-02 1 in 1 CARTON 05/01/2016 1 NDC:59078-309-01 45 mL in 1 TUBE; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part352 05/01/2016 Labeler - TONYMOLY CO.,LTD (688216798) Registrant - TONYMOLY CO.,LTD (688216798) Establishment Name Address ID/FEI Business Operations TONYMOLY CO.,LTD 688216798 manufacture(59078-309)