Label: neutrexin- Trimetrexate glucuronate injection, powder, lyophilized, for solution
-
Contains inactivated NDC Code(s)
NDC Code(s): 58178-020-01, 58178-020-10, 58178-020-50, 58178-021-01 - Packager: MedImmune Oncology, Inc.
- Category: HUMAN PRESCRIPTION DRUG LABEL
Drug Label Information
Updated May 11, 2006
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- N/A - Section Title Not Found In Database
- BOXED WARNING (What is this?)
-
DESCRIPTION
Neutrexin is the brand name for trimetrexate glucuronate. Trimetrexate, a 2,4-diaminoquinazoline, non-classical folate antagonist, is a synthetic inhibitor of the enzyme dihydrofolate reductase (DHFR). Neutrexin is available as a sterile lyophilized powder, containing trimetrexate glucuronate equivalent to either 200mg or 25mg of trimetrexate without any preservatives or excipients. The powder is reconstituted prior to intravenous infusion (see DOSAGE AND ADMINISTRATION, RECONSTITUTION AND DILUTION).
Trimetrexate glucuronate is chemically known as 2,4-diamino-5-methyl-6-[(3,4,5-trimethoxyanilino)methyl] quinazoline mono-D-glucuronate, and has the following structure:
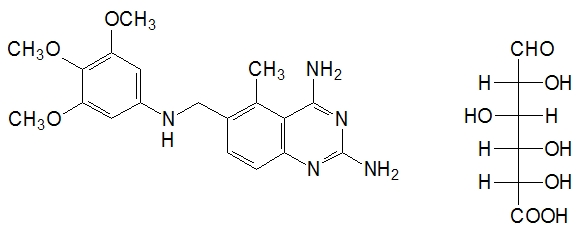
The empirical formula for trimetrexate glucuronate is C19H23N5O3• C6H10O7 with a molecular weight of 563.56. The active ingredient, trimetrexate free base, has an empirical formula of C19H23N5O3 with a molecular weight of 369.42. Trimetrexate glucuronate for injection is a pale greenish-yellow powder or cake. Trimetrexate glucuronate is soluble in water (>50 mg/mL), whereas trimetrexate free base is practically insoluble in water (<0.1 mg/mL). The pKa of trimetrexate free base in 50% methanol/water is 8.0. The logarithm10 of the partition coefficient of trimetrexate free base between octanol and water is 1.63.
-
CLINICAL PHARMACOLOGY
Mechanism of Action
In vitro studies have shown that trimetrexate is a competitive inhibitor of dihydrofolate reductase (DHFR) from bacterial, protozoan, and mammalian sources. DHFR catalyzes the reduction of intracellular dihydrofolate to the active coenzyme tetrahydrofolate. Inhibition of DHFR results in the depletion of this coenzyme, leading directly to interference with thymidylate biosynthesis, as well as inhibition of folate-dependent formyltransferases, and indirectly to inhibition of purine biosynthesis. The end result is disruption of DNA, RNA, and protein synthesis, with consequent cell death. Leucovorin (folinic acid) is readily transported into mammalian cells by an active, carrier‑mediated process and can be assimilated into cellular folate pools following its metabolism. In vitro studies have shown that leucovorin provides a source of reduced folates necessary for normal cellular biosynthetic processes. Because the Pneumocystis carinii organism lacks the reduced folate carrier-mediated transport system, leucovorin is prevented from entering the organism. Therefore, at concentrations achieved with therapeutic doses of trimetrexate plus leucovorin, the selective transport of trimetrexate, but not leucovorin, into the Pneumocystis carinii organism allows the concurrent administration of leucovorin to protect normal host cells from the cytotoxicity of trimetrexate without inhibiting the antifolate's inhibition of Pneumocystis carinii. It is not known if considerably higher doses of leucovorin would affect trimetrexate's effect on Pneumocystis carinii.
Microbiology
Trimetrexate inhibits, in a dose-related manner, in vitro growth of the trophozoite stage of rat Pneumocystis carinii cultured on human embryonic lung fibroblast cells. Trimetrexate concentrations between 3 and 54.1 μM were shown to inhibit the growth of trophozoites. Leucovorin alone at a concentration of 10 μM did not alter either the growth of the trophozoites or the anti-pneumocystis activity of trimetrexate. Resistance to trimetrexate's antimicrobial activity against Pneumocystis carinii has not been studied.
Pharmacokinetics
Trimetrexate pharmacokinetics were assessed in six patients with acquired immunodeficiency syndrome (AIDS) who had Pneumocystis carinii pneumonia (4 patients) or toxoplasmosis (2 patients). Trimetrexate was administered intravenously as a bolus injection at a dose of 30 mg/m2/day along with leucovorin 20 mg/m2 every 6 hours for 21 days. Trimetrexate clearance (mean ± SD) was 38 ± 15 mL/min/m2 and volume of distribution at steady state (Vdss) was 20 ± 8 L/m2. The plasma concentration time profile declined in a biphasic manner over 24 hours with a terminal half-life of 11 ± 4 hours.
The pharmacokinetics of trimetrexate without the concomitant administration of leucovorin have been evaluated in cancer patients with advanced solid tumors using various dosage regimens. The decline in plasma concentrations over time has been described by either biexponential or triexponential equations. Following the single-dose administration of 10 to 130 mg/m2 to 37 patients, plasma concentrations were obtained for 72 hours. Nine plasma concentration time profiles were described as biexponential. The alpha phase half-life was 57 ± 28 minutes, followed by a terminal phase with a half-life of 16 ± 3 hours. The plasma concentrations in the remaining patients exhibited a triphasic decline with half-lives of 8.6 ± 6.5 minutes, 2.4 ± 1.3 hours, and 17.8 ± 8.2 hours.
Trimetrexate clearance in cancer patients has been reported as 53 ± 41 mL/min (14 patients) and 32 ± 18 mL/min/m2 (23 patients) following single-dose administration. After a five-day infusion of trimetrexate to 16 patients, plasma clearance was 30 ± 8 mL/min/m2.
Renal clearance of trimetrexate in cancer patients has varied from about 4 ± 2 mL/min/m2 to 10 ± 6 mL/min/m2. Ten to 30% of the administered dose is excreted unchanged in the urine. Considering the free fraction of trimetrexate, active tubular secretion may possibly contribute to the renal clearance of trimetrexate. Renal clearance has been associated with urine flow, suggesting the possibility of tubular reabsorption as well.
The Vdss of trimetrexate in cancer patients after single-dose administration and for whom plasma concentrations were obtained for 72 hours was 36.9 ± 17.6 L/m2 (n=23) and 0.62 ± 0.24 L/kg (n=14). Following a constant infusion of trimetrexate for five days, Vdss was 32.8 ± 16.6 L/m2. The volume of the central compartment has been estimated as 0.17 ± 0.08 L/kg and 4.0 ± 2.9 L/m2.
There have been inconsistencies in the reporting of trimetrexate protein binding. The in vitro plasma protein binding of trimetrexate using ultrafiltration is approximately 95% over the concentration range of 18.75 to 1000 ng/mL. There is a suggestion of capacity limited binding (saturable binding) at concentrations greater than about 1000 ng/mL, with free fraction progressively increasing to about 9.3% as concentration is increased to 15 μg/mL. Other reports have declared trimetrexate to be greater than 98% bound at concentrations of 0.1 to 10 μg/mL; however, specific free fractions were not stated. The free fraction of trimetrexate also has been reported to be about 15 to 16% at a concentration of 60 ng/mL, increasing to about 20% at a trimetrexate concentration of 6 μg/mL.
Trimetrexate metabolism in man has not been characterized. Preclinical data strongly suggest that the major metabolic pathway is oxidative O-demethylation, followed by conjugation to either glucuronide or the sulfate. N-demethylation and oxidation is a related minor pathway. Preliminary findings in humans indicate the presence of a glucuronide conjugate with DHFR inhibition and a demethylated metabolite in urine.
The presence of metabolite(s) in human plasma following the administration of trimetrexate is suggested by the differences seen in trimetrexate plasma concentrations when measured by HPLC and a nonspecific DHFR inhibition assay. The profiles are similar initially, but diverge with time; concentrations determined by DHFR being higher than those determined by HPLC. This suggests the presence of one or more metabolites with DHFR inhibition activity. After intravenous administration of trimetrexate to humans, urinary recovery averaged about 40%, using a DHFR assay, in comparison to 10% urinary recovery as determined by HPLC, suggesting the presence of one or more metabolites that retain inhibitory activity against DHFR. Fecal recovery of trimetrexate over 48 hours after intravenous administration ranged from 0.09 to 7.6% of the dose as determined by DHFR inhibition and 0.02 to 5.2% of the dose as determined by HPLC.
The pharmacokinetics of trimetrexate have not been determined in patients with renal insufficiency or hepatic dysfunction.
-
INDICATIONS AND USAGE
Neutrexin (trimetrexate glucuronate for injection) with concurrent leucovorin administration (leucovorin protection) is indicated as an alternative therapy for the treatment of moderate-to-severe Pneumocystis carinii pneumonia (PCP) in immunocompromised patients, including patients with the acquired immunodeficiency syndrome (AIDS), who are intolerant of, or are refractory to, trimethoprim-sulfamethoxazole therapy or for whom trimethoprim-sulfamethoxazole is contraindicated.
This indication is based on the results of a randomized, controlled double-blind trial comparing Neutrexin with concurrent leucovorin protection (TMTX/LV) to trimethoprim‑sulfamethoxazole (TMP/SMX) in patients with moderate-to-severe Pneumocystis carinii pneumonia, as well as results of a Treatment IND. These studies are summarized below:
Neutrexin Comparative Study with TMP/SMX
This double-blind, randomized trial initiated by the AIDS Clinical Trials Group (ACTG) in 1988 was designed to compare the safety and efficacy of TMTX/LV to that of TMP/SMX for the treatment of histologically confirmed, moderate-to-severe PCP, defined as (A-a) baseline gradient >30 mmHg, in patients with AIDS.
Of the 220 patients with histologically confirmed PCP, 109 were randomized to receive TMTX/LV and 111 to TMP/SMX. Study patients randomized to TMTX/LV treatment were to receive 45 mg/m2 of TMTX daily for 21 days plus 20 mg/m2 of LV every 6 hours for 24 days. Those randomized to TMP/SMX were to receive 5 mg/kg TMP plus 25 mg/kg SMX four times daily for 21 days.
Response to therapy, defined as alive and off ventilatory support at completion of therapy, with no change in anti-pneumocystis therapy, or addition of supraphysiologic doses of steroids, occurred in fifty percent of patients in each treatment group.
The observed mortality in the TMTX/LV treatment group was approximately twice that in the TMP/SMX treatment group (95% CI: 0.99 - 4.11). Thirty of 109 (27%) patients treated with TMTX/LV and 18 of 111 (16%) patients receiving TMP/SMX died during the 21-day treatment course or 4-week follow-up period. Twenty-seven of 30 deaths in the TMTX/LV arm were attributed to PCP; all 18 deaths in the TMP/SMX arm were attributed to PCP.
A significantly smaller proportion of patients who received TMTX/LV compared to TMP/SMX failed therapy due to toxicity (10% vs. 25%), and a significantly greater proportion of patients failed due to lack of efficacy (40% vs. 24%). Six patients (12%) who responded to TMTX/LV relapsed during the one-month follow-up period; no patient responding to TMP/SMX relapsed during this period. Information is not available as to whether these patients received prophylaxis therapy for PCP.
Treatment IND
The FDA granted a Treatment IND for Neutrexin with leucovorin protection in February 1988 to make Neutrexin therapy available to HIV-infected patients with histologically confirmed PCP who had disease refractory to or who were intolerant of TMP/SMX and/or intravenous pentamidine.
Over 500 physicians in the United States participated in the Treatment IND. Of the first 753 patients enrolled, 577 were evaluable for efficacy. Of these, 227 patients were intolerant of both TMP/SMX and pentamidine (IST - patients intolerant of both standard therapies), 146 were intolerant of one therapy and refractory to the other (RIST - patients refractory to one therapy and intolerant of the other) and 204 were refractory to both therapies (RST - refractory to both standard therapies). This was a very ill patient population; 38% required ventilatory support at entry (Table 1). These studies did not have concurrent control groups.
TABLE 1 TREATMENT IND Baseline Characteristics IST
(n = 227)RIST
(n = 146)RST
(n = 204)TOTAL
(n = 577)Ventilatory Support Required
n (%)39 (17) 50 (34) 129 (63) 218 (38) Median Days on Standard Therapy 10 12 16 14 First Episode of PCP
n (%)104 (46) 103 (71) 190 (93) 397 (69) The overall survival rate one month after completion of TMTX/LV as salvage therapy was 48%. Patients who had not responded to treatment with both TMP/SMX and pentamidine, of whom 63% required mechanical ventilation at entry, achieved a survival rate of 25% following treatment with TMTX/LV. Survival was 67% in patients who were intolerant to both TMP/SMX and pentamidine (Table 2).
TABLE 2 TREATMENT IND Survival Rate One Month After Completion of Neutrexin Therapy IST RIST RST All Patients
153/227 (67%) 73/146 (50%) 50/204 (25%) Baseline Ventilatory Support
9/39 (23%) 15/50 (30%) 18/129 (14%) No Baseline Ventilatory Support 144/188 (77%) 58/96 (60%) 32/75 (43%) In the Treatment IND, 12% of the patients discontinued Neutrexin therapy (with leucovorin protection) for toxicity.
- CONTRAINDICATIONS
-
WARNINGS
Neutrexin (trimetrexate glucuronate for injection) must be used with concurrent leucovorin to avoid potentially serious or life-threatening complications including bone marrow suppression, oral and gastrointestinal mucosal ulceration, and renal and hepatic dysfunction. Leucovorin therapy must extend for 72 hours past the last dose of Neutrexin. Patients should be informed that failure to take the recommended dose and duration of leucovorin can lead to fatal toxicity. Patients should be closely monitored for the development of serious hematologic adverse reactions (see PRECAUTIONS and DOSAGE AND ADMINISTRATION).
Neutrexin can cause fetal harm when administered to a pregnant woman. Trimetrexate has been shown to be fetotoxic and teratogenic in rats and rabbits. Rats administered 1.5 and 2.5 mg/kg/day intravenously on gestational days 6-15 showed substantial postimplantation loss and severe inhibition of maternal weight gain. Trimetrexate administered intravenously to rats at 0.5 and 1.0 mg/kg/day on gestational days 6-15 retarded normal fetal development and was teratogenic. Rabbits administered trimetrexate intravenously at daily doses of 2.5 and 5.0 mg/kg/day on gestational days 6-18 resulted in significant maternal and fetal toxicity. In rabbits, trimetrexate at 0.1 mg/kg/day was teratogenic in the absence of significant maternal toxicity. These effects were observed using doses 1/20 to 1/2 the equivalent human therapeutic dose based on a mg/m2 basis. Teratogenic effects included skeletal, visceral, ocular, and cardiovascular abnormalities. If Neutrexin is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus. Women of childbearing potential should be advised to avoid becoming pregnant.
-
PRECAUTIONS
General
Patients receiving Neutrexin (trimetrexate glucuronate for injection) may experience severe hematologic, hepatic, renal, and gastrointestinal toxicities. Caution should be used in treating patients with impaired hematologic, renal, or hepatic function. Patients who require concomitant therapy with nephrotoxic, myelosuppressive, or hepatotoxic drugs should be treated with Neutrexin at the discretion of the physician and monitored carefully. To allow for full therapeutic doses of Neutrexin, treatment with zidovudine should be discontinued during Neutrexin therapy.
Neutrexin-associated myelosuppression, stomatitis, and gastrointestinal toxicities generally can be ameliorated by adjusting the dose of leucovorin. Mild elevations in transaminases and alkaline phosphatase have been observed with Neutrexin administration and are usually not cause for modification of Neutrexin therapy (see DOSAGE AND ADMINISTRATION). Seizures have been reported rarely (< 1%) in AIDS patients receiving Neutrexin; however, a causal relationship has not been established. Trimetrexate is a known inhibitor of histamine metabolism. Hypersensitivity/allergic type reactions including but not limited to rash, chills/rigors, fever, diaphoresis and dypsnea have occurred with trimetrexate primarily when it is administered as a bolus infusion or at doses higher than those recommended for PCP, and most frequently in combination with 5FU and leucovorin. In rare cases, anaphylactoid reactions, including acute hypotension and loss of consciousness have occurred. Neutrexin infusion should be permanently discontinued in all patients with severe hypersensitivity reactions. Epinephrine should be available for treatment of acute allergic symptoms.
Neutrexin has not been evaluated clinically for the treatment of concurrent pulmonary conditions such as bacterial, viral, or fungal pneumonia or mycobacterial diseases. In vitro activity has been observed against Toxoplasma gondii, Mycobacterium avium complex, gram positive cocci, and gram negative rods. If clinical deterioration is observed in patients, they should be carefully evaluated for other possible causes of pulmonary disease and treated with additional agents as appropriate.
Laboratory Tests
Patients receiving Neutrexin with leucovorin protection should be seen frequently by a physician. Blood tests to assess the following parameters should be performed at least twice a week during therapy: hematology (absolute neutrophil counts [ANC], platelets), renal function (serum creatinine, BUN), and hepatic function (AST, ALT, alkaline phosphatase).
Drug Interactions
Since trimetrexate is metabolized by a P450 enzyme system, drugs that induce or inhibit this drug metabolizing enzyme system may elicit important drug-drug interactions that may alter trimetrexate plasma concentrations. Agents that might be coadministered with trimetrexate in AIDS patients for other indications that could elicit this activity include erythromycin, rifampin, rifabutin, ketoconazole, and fluconazole. In vitro perfusion of isolated rat liver has shown that cimetidine caused a significant reduction in trimetrexate metabolism and that acetaminophen altered the relative concentration of trimetrexate metabolites possibly by competing for sulfate metabolites. Based on an in vitro rat liver model, nitrogen substituted imidazole drugs (clotrimazole, ketoconazole, miconazole) were potent, non-competitive inhibitors of trimetrexate metabolism. Patients medicated with these drugs and trimetrexate should be carefully monitored.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenesis
Long term studies in animals to evaluate the carcinogenic potential of trimetrexate have not been performed.
Mutagenesis
Trimetrexate was not mutagenic when tested using the standard Ames Salmonella mutagenicity assay with and without metabolic activation. Trimetrexate did not induce mutations in Chinese hamster lung cells or sister-chromatid exchange in Chinese hamster ovary cells. Trimetrexate did induce an increase in the chromosomal aberration frequency of cultured Chinese hamster lung cells; however, trimetrexate showed no clastogenic activity in a mouse micronucleus assay.
Nursing Mothers
It is not known if trimetrexate is excreted in human milk. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from trimetrexate, it is recommended that breast feeding be discontinued if the mother is treated with Neutrexin.
Pediatric Use
The safety and effectiveness of Neutrexin for the treatment of histologically confirmed PCP has not been established for patients under 18 years of age. Two children, ages 15 months and 9 months, were treated with trimetrexate and leucovorin using a dose of 45 mg/m2 of trimetrexate per day for 21 days and 20 mg/m2 of leucovorin every 6 hours for 24 days. There were no serious or unexpected adverse effects.
-
ADVERSE REACTIONS
Because many patients who participated in clinical trials of Neutrexin (trimetrexate glucuronate for injection) had complications of advanced HIV disease, it is difficult to distinguish adverse events caused by Neutrexin from those resulting from underlying medical conditions.
Table 3 lists the adverse events that occurred in ≥ 1% of the patients who participated in the Comparative Study of Neutrexin plus leucovorin versus TMP/SMX.
TABLE 3 NEUTREXIN COMPARATIVE TRIAL Comparison of Adverse Events Reported for ≥ 1% of Patients Adverse Events Number and Percent (%) of Patients with Adverse Events TMTX/LV
(n = 109)TMP/SMX
(n = 111)Non-Laboratory Adverse Events: Fever 9 (8.3) 14 (12.6) Rash/Pruritus 6 (5.5) 14 (12.6) Nausea/Vomiting 5 (4.6)* 15 (13.5)* Confusion 3 (2.8) 3 (2.7) Fatigue 2 (1.8) 0 (0.0) Hematologic Toxicity: Thrombocytopenia (<75,000/mm3) 33 (30.3) 37 (33.3) Anemia (Hgb <8 g/dL) 11 (10.1) 17 (15.3) Neutropenia (<1000/mm3) 8 (7.3) 10 (9.0) Hepatotoxicity: Increased AST (>5 x ULN†) 15 (13.8) 10 (9.0) Increased ALT (>5 x ULN) 12 (11.0) 13 (11.7) Increased Alkaline Phosphatase (>5 x ULN) 5 (4.6) 3 (2.7) Increased Bilirubin (2.5 x ULN) 2 (1.8) 1 (0.9) Renal: Increased Serum Creatinine (>3 x ULN) 1 (0.9) 2 (1.8) Electrolyte Imbalance: Hyponatremia 5 (4.6) 10 (9.0) Hypocalcemia 2 (1.8) 0 (0.0) No. of Patients With at Least one Adverse Event‡ 58 (53.2) 60 (54.1) Laboratory toxicities were generally manageable with dose modification of
trimetrexate/leucovorin (see DOSAGE AND ADMINISTRATION).
Table 4 lists the adverse events resulting in discontinuation of study therapy in the Neutrexin Comparative Study with TMP/SMX. Twenty-nine percent of the patients on the TMP/SMX arm discontinued therapy due to adverse events compared to 10% of the patients treated with TMTX/LV (p < 0.001).
TABLE 4 NEUTREXIN COMPARATIVE TRIAL Adverse Events Resulting in Discontinuation of Therapy - *
- Patients could discontinue therapy due to more than one toxicity; therefore the sum exceeds number of patients who discontinued due to toxicity
- †
- Patient discontinued TMTX/LV due to seizure, though causal relationship could not be established.
- ‡
- ULN = Upper limit of normal range
- §
- Statistically significant difference between treatment groups (Chi-square: p < 0.001)
Adverse Events Number and Percent (%) of Patients Discontinued for Adverse Events*
TMTX/LV
(n = 109)TMP/SMX
(n = 111)Non-Laboratory Adverse Events: Rash/Pruritus 3 (2.8) 5 (4.5) Fever 2 (1.8) 4 (3.6) Nausea/Vomiting 1 (0.9) 8 (7.2) Neurologic Toxicity 1 (0.9)† 2 (1.8) Hematologic Toxicity: Neutropenia (<1000/mm3) 4 (3.7) 6 (5.4) Thrombocytopenia (<75,000/mm3) 0 (0.0) 4 (3.6) Anemia (Hgb <8 g/dL) 0 (0.0) 4 (3.6) Hepatotoxicity: Increased AST (>5 x ULN‡) 3 (2.8) 9 (8.1) Increased ALT (>5 x ULN) 1 (0.9) 4 (3.6) Increased Alkaline Phosphatase (>5 x ULN) 0 (0.0) 1 (0.9) Electrolyte Imbalance: Hyponatremia 0 (0.0) 3 (2.7) No. of Patients Discontinuing Therapy Due to an Adverse Event* 11 (10.1)§ 32 (28.8)§ Hematologic toxicity was the principal dose-limiting side effect.
-
OVERDOSAGE
Neutrexin (trimetrexate glucuronate for injection) administered without concurrent
leucovorin can cause lethal complications. There has been no extensive experience in humans receiving single intravenous doses of trimetrexate greater than 90 mg/m2/day with concurrent leucovorin. The toxicities seen at this dose were primarily hematologic. In the event of overdose, Neutrexin should be stopped and leucovorin should be administered at a dose of 40 mg/m2 every 6 hours for 3 days. The LD50 of intravenous trimetrexate in mice is 62 mg/kg (186 mg/m2).
-
DOSAGE AND ADMINISTRATION
Caution: Neutrexin (trimetrexate glucuronate for injection) must be administered with concurrent leucovorin (leucovorin protection) to avoid potentially serious or life-threatening toxicities. Leucovorin therapy must extend for 72 hours past the last dose of Neutrexin.
Neutrexin (trimetrexate glucuronate for injection) is administered at a dose of 45 mg/m2 once daily by intravenous infusion over 60 minutes. Leucovorin must be administered daily during treatment with Neutrexin and for 72 hours past the last dose of Neutrexin. Leucovorin may be administered intravenously at a dose of 20 mg/m2 over 5 to 10 minutes every 6 hours for a total daily dose of 80 mg/m2, or orally as 4 doses of 20 mg/m2 spaced equally throughout the day. The oral dose should be rounded up to the next higher 25 mg increment. The recommended course of therapy is 21 days of Neutrexin and 24 days of leucovorin.
Neutrexin and leucovorin may alternatively be dosed on a mg/kg basis, depending on the patient’s body weight, using the conversion factors shown in the table below:
Body Weight (kg) Neutrexin Dose (mg/kg/day) Leucovorin Dose (mg/kg/qid) <50 1.5 0.6 50-80 1.2 0.5 >80 1.0 0.5 Dosage Modifications
Hematologic toxicity: Neutrexin (trimetrexate glucuronate for injection) and leucovorin doses should be modified based on the worst hematologic toxicity according to the following table. If leucovorin is given orally, doses should be rounded up to the next higher 25 mg increment.
TABLE 5 DOSE MODIFICATIONS FOR HEMATOLOGIC TOXICITY - *
- If Grade 4 hematologic toxicity occurs prior to Day 10, Neutrexin should be discontinued. Leucovorin (40 mg/m2, q6h) should be administered for an additional 72 hours. If Grade 4 hematologic toxicity occurs at Day 10 or later, Neutrexin may be held up to 96 hours to allow counts to recover. If counts recover to Grade 3 within 96 hours, Neutrexin should be administered at a dose of 22 mg/m2 and leucovorin maintained at 40 mg/m2, q6h. When counts recover to Grade 2 toxicity, Neutrexin dose may be increased to 45 mg/m2, but the leucovorin dose should be maintained at 40 mg/m2 for the duration of treatment. If counts do not improve to ≤ Grade 3 toxicity within 96 hours, Neutrexin should be discontinued. Leucovorin at a dose of 40 mg/m2, q6h should be administered for 72 hours following the last dose of Neutrexin.
Recommended Dosages of Toxicity Grade Neutrophils
(Polys and Bands)
Platelets Neutrexin Leucovorin 1 >1000/mm3 >75,000/mm3 45 mg/m2 once daily 20 mg/m2 every 6 hours 2 750-1000/mm3 50,000-75,000/mm3 45 mg/m2 once daily 40 mg/m2 every 6 hours 3 500-749/mm3 25,000-49,999/mm3 22 mg/m2 once daily 40 mg/m2 every 6 hours 4 <500/mm3 <25,000/mm3 Day 1-9 Discontinue
Day 10-21 Interrupt up to 96 hours*40 mg/m2 every 6 hours Hepatic toxicity: Transient elevations of transaminases and alkaline phosphatase have been observed in patients treated with Neutrexin. Interruption of treatment is advisable if transaminase levels or alkaline phosphatase levels increase to >5 times the upper limit of normal range.
Renal toxicity: Interruption of Neutrexin is advisable if serum creatinine levels increase to > 2.5 mg/dL and the elevation is considered to be secondary to Neutrexin.
Other toxicities: Interruption of treatment is advisable in patients who experience severe mucosal toxicity that interferes with oral intake. Treatment should be discontinued for fever (oral temperature ≥ 105°F/40.5°C) that cannot be controlled with antipyretics.
Leucovorin therapy must extend for 72 hours past the last dose of Neutrexin.
-
RECONSTITUTION AND DILUTION
Each vial of Neutrexin (trimetrexate glucuronate for injection) should be reconstituted in accordance with labeled instructions with either 5% Dextrose Injection, USP, or Sterile Water for Injection, USP, to yield a concentration of 12.5 mg of trimetrexate per mL (complete dissolution should occur within 30 seconds). The reconstituted product will appear as a pale greenish-yellow solution and must be inspected visually prior to dilution. Do not use if cloudiness or precipitate is observed. Neutrexin should not be reconstituted with solutions containing either chloride ion or leucovorin, since precipitation occurs instantly.
After reconstitution, the solution should be used immediately; however, the solution is stable for 6 hours at room temperature (20 to 25ºC), or 24 hours under refrigeration (2-8°C).
Prior to administration, the reconstituted solution should be further diluted with 5% Dextrose Injection, USP, to yield a final concentration of 0.25 to 2 mg of trimetrexate per mL. The diluted solution should be administered by intravenous infusion over 60 minutes. Neutrexin should not be mixed with solutions containing either chloride ion or leucovorin, since precipitation occurs instantly. The diluted solution is stable under refrigeration or at room temperature for up to 24 hours. Do not freeze. Discard any unused portion after 24 hours. The intravenous line must be flushed thoroughly with at least 10 mL of 5% Dextrose Injection, USP, before and after administering Neutrexin.
Leucovorin protection may be administered prior to or following Neutrexin. In either case, the intravenous line must be flushed thoroughly with at least 10 mL of 5% Dextrose Injection, USP. Leucovorin calcium for injection should be diluted according to the instructions in the leucovorin package insert, and administered over 5 to 10 minutes every 6 hours.
Caution: Parenteral products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Neutrexin forms a precipitate instantly upon contact with chloride ion or leucovorin, therefore it should not be added to solutions containing sodium chloride or other anions. Neutrexin and leucovorin solutions must be administered separately. Intravenous lines should be flushed with at least 10 mL of 5% Dextrose Injection, USP, between Neutrexin and leucovorin infusions.
- HANDLING AND DISPOSAL
-
HOW SUPPLIED
Neutrexin (trimetrexate glucuronate for injection) is supplied as a sterile lyophilized powder in either 5 mL or 30 mL vials. Each 5 mL vial contains trimetrexate glucuronate equivalent to 25 mg of trimetrexate. Each 30 mL vial contains trimetrexate glucuronate equivalent to 200 mg of trimetrexate. The 5 mL vials are packaged and available in two market presentations as listed below:
10 Pack - 10 vials in a white chip-board carton (NDC 58178-020-10)
50 Pack - 2 trays of 25 vials per shrink-wrapped tray (NDC 58178-020-50)
The 30 mL vials are packaged and available as listed below:
Single Pack - 1 vial (NDC 58178-021-01)
Store at controlled room temperature 20° to 25°C (68° to 77°F). Protect from exposure to light.
U.S. Patents 4,376,858; 4,694,007; 6,017,922
-
REFERENCES
- AMA Council Report. Guidelines for Handling Parenteral Antineoplastics. Journal of the American Medical Association March 15, 1985.
- Clinical Oncological Society of Australia: Guidelines and Recommendations for Safe Handling of Antineoplastic Agents. Medical Journal of Australia 1: 426-428, 1983.
- Jones RB, et al. Safe Handling of Chemotherapeutic Agents: A Report from the Mount Sinai Medical Center. CA - A Cancer Journal for Clinicians Sept/Oct, 258-263, 1983.
- American Society of Hospital Pharmacists Technical Assistance Bulletin on Handling Cytotoxic Drugs in Hospitals. American Journal of Hospital Pharmacy 42: 131-137, 1985.
- OSHA Work Practice Guidelines for Personnel Dealing with Cytotoxic (Antineoplastic) Drugs. American Journal of Hospital Pharmacy 43: 1193-1204, 1986.
Manufactured by: Marketed by:
Ben Venue Laboratories, Inc. MedImmune Oncology, Inc.
Bedford, OH 44146 a subsidiary of MedImmune Inc.
Gaithersburg, MD 20878
Or: 1-877-633-4411
MedImmune Pharma B.V.
6545 CG Nijmegen
The Netherlands
© 2000, MedImmune Oncology, Inc.
N-LB3020 PH
-
INGREDIENTS AND APPEARANCE
NEUTREXIN
trimetrexate glucuronate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58178-021 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength trimetrexate glucuronate (UNII: L137U4A79K) (trimetrexate - UNII:UPN4ITI8T4) 200 mg in 16 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58178-021-01 16 mL in 1 VIAL, SINGLE-USE NEUTREXIN
trimetrexate glucuronate injection, powder, lyophilized, for solutionProduct Information Product Type HUMAN PRESCRIPTION DRUG Item Code (Source) NDC:58178-020 Route of Administration INTRAVENOUS Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength trimetrexate glucuronate (UNII: L137U4A79K) (trimetrexate - UNII:UPN4ITI8T4) 25 mg in 2 mL Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:58178-020-10 10 in 1 CARTON 1 NDC:58178-020-01 2 mL in 1 VIAL, SINGLE-USE 2 NDC:58178-020-50 50 in 1 CARTON 2 NDC:58178-020-01 2 mL in 1 VIAL, SINGLE-USE 3 NDC:58178-020-01 2 mL in 1 VIAL, SINGLE-USE Labeler - MedImmune Oncology, Inc.
