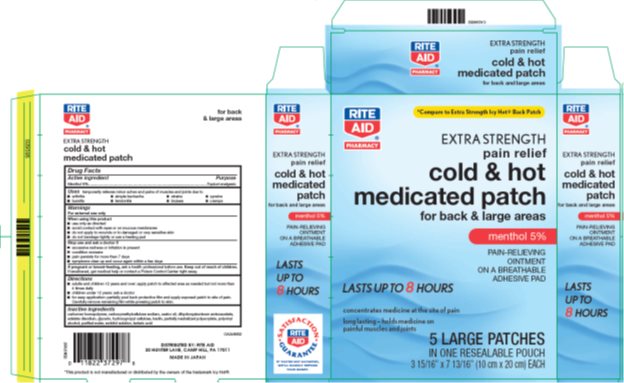
Label: RITE AID COLD AND HOT MEDICATED- menthol patch
- NDC Code(s): 11822-3729-7
- Packager: Rite Aid Corporation
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated February 7, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active ingredient
- Purpose
- Uses
-
Warnings
For external use only
When using this product
- use only as directed
- avoid contact with eyes or on mucous membranes
- do not apply to wounds or to damaged or very sensitive skin
- do not bandage tightly or use a heating pad
-
Directions
- adults and children 12 years and over: apply patch to affected area as needed but no more than 4 times daily
- children under 12 years: ask a doctor
- for easy application: grasp both ends of pad firmly, pull at both ends. Stretch pad until backing separates. Remove protective film while applying pad directly to site of pain.
- Inactive ingredients
- Package/Label Principal Display Panel
-
INGREDIENTS AND APPEARANCE
RITE AID COLD AND HOT MEDICATED
menthol patchProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:11822-3729 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength MENTHOL, UNSPECIFIED FORM (UNII: L7T10EIP3A) (MENTHOL, UNSPECIFIED FORM - UNII:L7T10EIP3A) MENTHOL, UNSPECIFIED FORM 50 g Inactive Ingredients Ingredient Name Strength CARBOMER HOMOPOLYMER TYPE B (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: HHT01ZNK31) CARBOXYMETHYLCELLULOSE SODIUM, UNSPECIFIED FORM (UNII: K679OBS311) CASTOR OIL (UNII: D5340Y2I9G) DIHYDROXYALUMINUM AMINOACETATE (UNII: DO250MG0W6) EDETATE DISODIUM (UNII: 7FLD91C86K) GLYCERIN (UNII: PDC6A3C0OX) HYDROXYPROPYL CELLULOSE, UNSPECIFIED (UNII: 9XZ8H6N6OH) KAOLIN (UNII: 24H4NWX5CO) POLYACRYLIC ACID (250000 MW) (UNII: 9G2MAD7J6W) POLYVINYL ALCOHOL, UNSPECIFIED (UNII: 532B59J990) WATER (UNII: 059QF0KO0R) SORBITOL (UNII: 506T60A25R) TARTARIC ACID (UNII: W4888I119H) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:11822-3729-7 1 in 1 CARTON 06/29/2010 1 5 in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part348 06/29/2010 Labeler - Rite Aid Corporation (014578892)