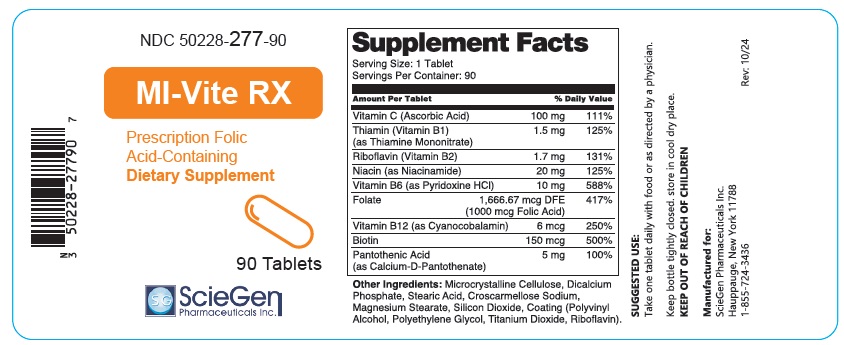
Label: MI-VITE RX- ascorbic acid, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, and calcium-d-pantothenate tablet
- NHRIC Code(s): 50228-277-90
- Packager: ScieGen Pharmaceuticals, Inc
- Category: DIETARY SUPPLEMENT
Drug Label Information
Updated October 30, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- STATEMENT OF IDENTITY
- HEALTH CLAIM
-
DOSAGE & ADMINISTRATION
Supplement Facts Serving Size: 1 Tablet
Servings Per Container: 90Amount Per Tablet % Daily Value Vitamin C (Ascorbic Acid) 100mg 111% Thiamin (Vitamin B1)
(as Thiamine Mononitrate)1.5 mg 125% Riboflavin (Vitamin B2) 1.7 mg 131% Niacin (as Niacinamide) 20 mg 125% Vitamin B6 (as Pyridoxine HCI) 10 mg 588% Folate 1,666.67 mcg DFE
(1000 mcg Folic Acid)417% Vitamin B12 (as Cyanocobalamin) 6 mcg 250% Biotin 150 mcg 500% Pantothenic Acid
(as Calcium-D-Pantothenate)5mg 100% Other Ingredients: Microcrystalline Cellulose, Dicalcium Phosphate, Stearic Acid, Croscarmellose Sodium, Magnesium Stearate, Silicon Dioxide, Coating (Polyvinyl Alcohol, Polyethylene Glycol, Titanium Dioxide, Riboflavin).
SUGGESTED USE:
Take one tablet daily with food or as directed by a physician.Keep bottle tightly closed. store in cool dry place.
KEEP OUT OF REACH OF CHILDRENManufactured for:
ScieGen Pharmaceuticals Inc.
Hauppauge, New York 11788
1-855-724-3436Rev: 10/24
- HEALTH CLAIM
- PRINCIPAL DISPLAY PANEL - 90 Tablet Bottle Label
-
INGREDIENTS AND APPEARANCE
MI-VITE RX
ascorbic acid, thiamine mononitrate, riboflavin, niacinamide, pyridoxine hydrochloride, folic acid, cyanocobalamin, biotin, and calcium-d-pantothenate tabletProduct Information Product Type DIETARY SUPPLEMENT Item Code (Source) NHRIC:50228-277 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ASCORBIC ACID (UNII: PQ6CK8PD0R) (ASCORBIC ACID - UNII:PQ6CK8PD0R) ASCORBIC ACID 100 mg THIAMINE MONONITRATE (UNII: 8K0I04919X) (THIAMINE ION - UNII:4ABT0J945J) THIAMINE 1.5 mg RIBOFLAVIN (UNII: TLM2976OFR) (RIBOFLAVIN - UNII:TLM2976OFR) RIBOFLAVIN 1.7 mg NIACINAMIDE (UNII: 25X51I8RD4) (NIACINAMIDE - UNII:25X51I8RD4) NIACINAMIDE 20 mg PYRIDOXINE HYDROCHLORIDE (UNII: 68Y4CF58BV) (PYRIDOXINE - UNII:KV2JZ1BI6Z) PYRIDOXINE 10 mg FOLIC ACID (UNII: 935E97BOY8) (FOLIC ACID - UNII:935E97BOY8) FOLIC ACID 1666.67 ug CYANOCOBALAMIN (UNII: P6YC3EG204) (CYANOCOBALAMIN - UNII:P6YC3EG204) CYANOCOBALAMIN 6 ug BIOTIN (UNII: 6SO6U10H04) (BIOTIN - UNII:6SO6U10H04) BIOTIN 150 ug PANTOTHENIC ACID (UNII: 19F5HK2737) (PANTOTHENIC ACID - UNII:19F5HK2737) PANTOTHENIC ACID 5 mg Inactive Ingredients Ingredient Name Strength MICROCRYSTALLINE CELLULOSE (UNII: OP1R32D61U) DICALCIUM PHOSPHATE (UNII: L11K75P92J) STEARIC ACID (UNII: 4ELV7Z65AP) CROSCARMELLOSE SODIUM (UNII: M28OL1HH48) MAGNESIUM STEARATE (UNII: 70097M6I30) SILICON DIOXIDE (UNII: ETJ7Z6XBU4) POLYVINYL ALCOHOL (UNII: 532B59J990) POLYETHYLENE GLYCOL, UNSPECIFIED (UNII: 3WJQ0SDW1A) TITANIUM DIOXIDE (UNII: 15FIX9V2JP) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NHRIC:50228-277-90 90 in 1 BOTTLE Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date DIETARY SUPPLEMENT 10/31/2024 Supplement Facts Serving Size : Serving per Container : Amount Per Serving % Daily Value color scoring 1 shape size (solid drugs) 10 mm Labeler - ScieGen Pharmaceuticals, Inc (079391286) Establishment Name Address ID/FEI Business Operations ScieGen Pharmaceuticals, Inc 079391286 manufacture(50228-277)