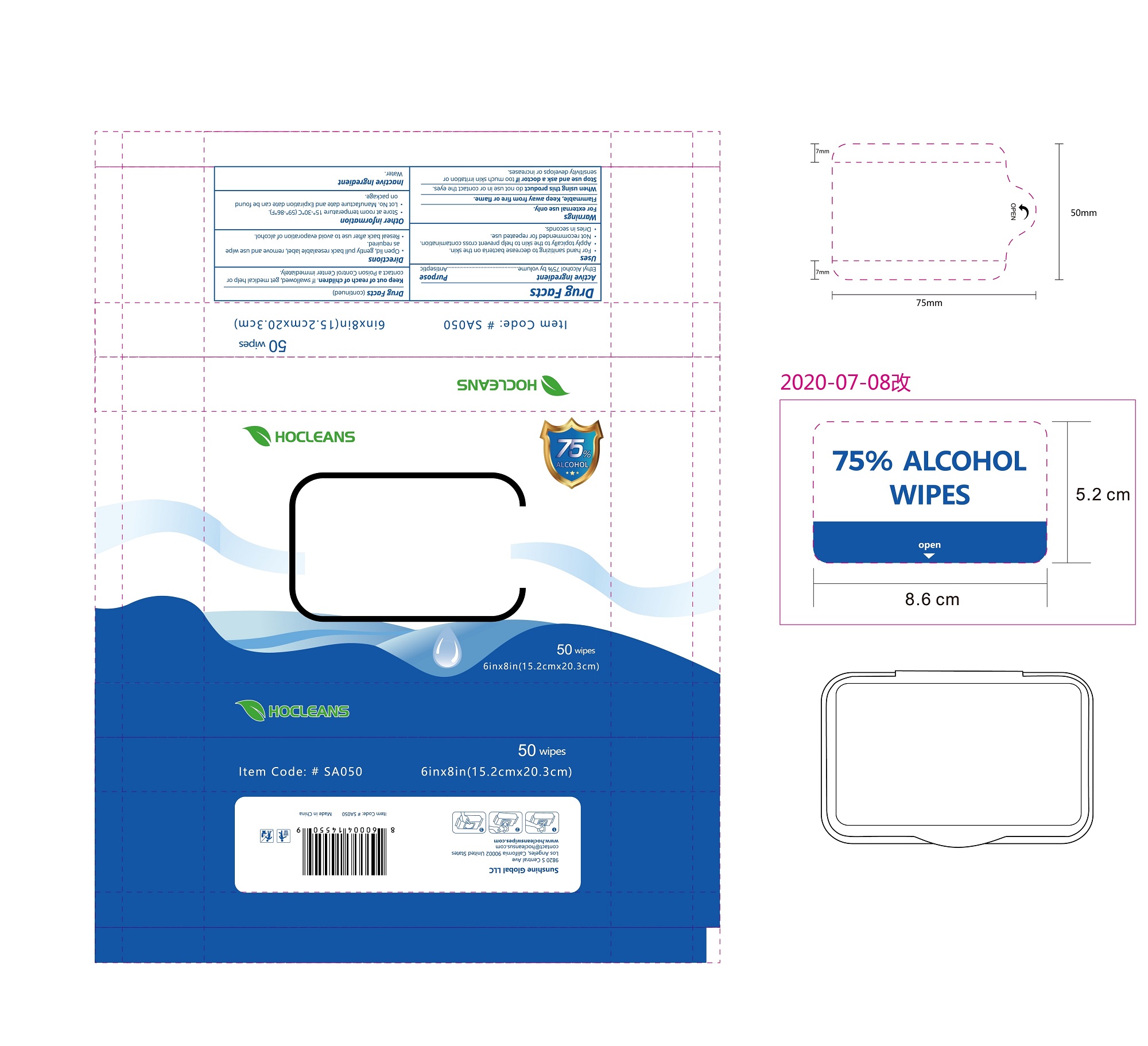
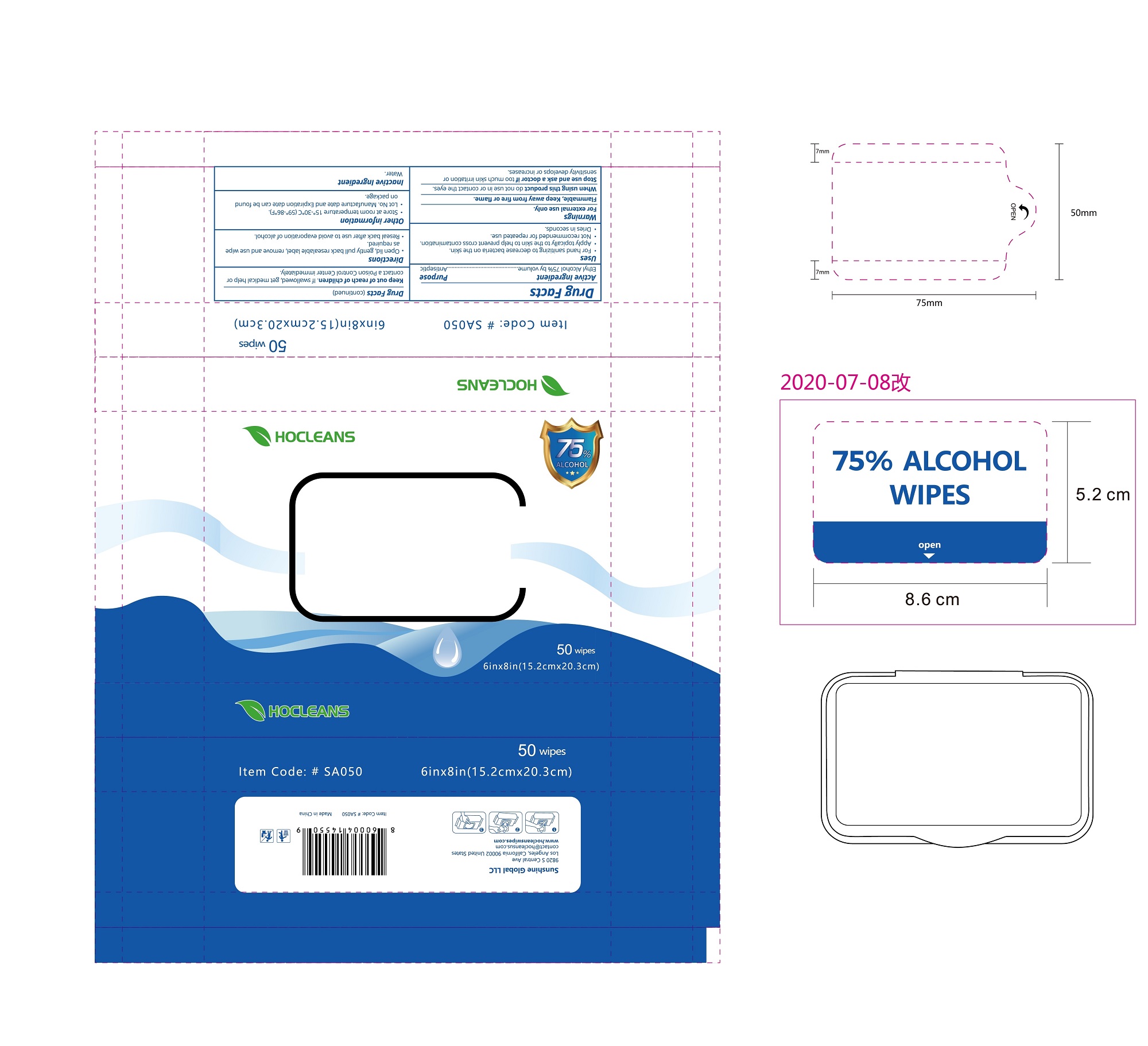
Label: ALCOHOL WIPES- alcohol cloth
-
NDC Code(s):
75109-503-01,
75109-503-02,
75109-503-03,
75109-503-04, view more75109-503-05, 75109-503-06, 75109-503-07, 75109-503-08, 75109-503-09, 75109-503-10, 75109-503-11, 75109-503-12, 75109-503-13, 75109-503-14, 75109-503-15, 75109-503-16, 75109-503-17, 75109-503-18, 75109-503-19, 75109-503-20, 75109-503-21, 75109-503-22, 75109-503-23, 75109-503-24, 75109-503-25, 75109-503-26
- Packager: Kangna (Zhejiang) Medical Supplies Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: Most OTC drugs are not reviewed and approved by FDA, however they may be marketed if they comply with applicable regulations and policies. FDA has not evaluated whether this product complies.
Drug Label Information
Updated January 14, 2022
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- USE
- Warning
- Directions
- Inactive ingredients
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
ALCOHOL WIPES
alcohol clothProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:75109-503 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 75 mL in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:75109-503-01 100 in 1 BOX 06/21/2020 1 2 mL in 1 POUCH; Type 0: Not a Combination Product 2 NDC:75109-503-02 50 in 1 BOX 06/21/2020 2 2.8 mL in 1 POUCH; Type 0: Not a Combination Product 3 NDC:75109-503-03 5 in 1 PACKAGE 06/21/2020 3 18.9 mL in 1 POUCH; Type 0: Not a Combination Product 4 NDC:75109-503-04 10 in 1 PACKAGE 06/21/2020 4 37.8 mL in 1 POUCH; Type 0: Not a Combination Product 5 NDC:75109-503-05 25 in 1 PACKAGE 06/21/2020 5 94.5 mL in 1 POUCH; Type 0: Not a Combination Product 6 NDC:75109-503-06 50 in 1 PACKAGE 06/21/2020 6 189 mL in 1 POUCH; Type 0: Not a Combination Product 7 NDC:75109-503-07 50 in 1 CASE 06/21/2020 7 24 in 1 PACKAGE 7 189 mL in 1 POUCH; Type 0: Not a Combination Product 8 NDC:75109-503-08 80 in 1 PACKAGE 06/21/2020 8 302.4 mL in 1 POUCH; Type 0: Not a Combination Product 9 NDC:75109-503-09 100 in 1 PACKAGE 06/21/2020 9 378 mL in 1 POUCH; Type 0: Not a Combination Product 10 NDC:75109-503-10 40 in 1 CANISTER 06/21/2020 10 181.44 mL in 1 POUCH; Type 0: Not a Combination Product 11 NDC:75109-503-11 100 in 1 CANISTER 06/21/2020 11 408.24 mL in 1 POUCH; Type 0: Not a Combination Product 12 NDC:75109-503-12 70 in 1 CANISTER 06/21/2020 12 126.224 mL in 1 POUCH; Type 0: Not a Combination Product 13 NDC:75109-503-13 70 in 1 CANISTER 06/21/2020 13 141.12 mL in 1 POUCH; Type 0: Not a Combination Product 14 NDC:75109-503-14 100 in 1 CANISTER 06/21/2020 14 272.16 mL in 1 POUCH; Type 0: Not a Combination Product 15 NDC:75109-503-15 150 in 1 CANISTER 06/21/2020 15 235.2 mL in 1 POUCH; Type 0: Not a Combination Product 16 NDC:75109-503-16 160 in 1 CANISTER 06/21/2020 16 333.8496 mL in 1 POUCH; Type 0: Not a Combination Product 17 NDC:75109-503-17 100 in 1 CANISTER 06/21/2020 17 186.2 mL in 1 POUCH; Type 0: Not a Combination Product 18 NDC:75109-503-18 800 in 1 PACKAGE 06/21/2020 18 1515.136 mL in 1 POUCH; Type 0: Not a Combination Product 19 NDC:75109-503-19 1200 in 1 PACKAGE 06/21/2020 19 2272.704 mL in 1 POUCH; Type 0: Not a Combination Product 20 NDC:75109-503-20 10 in 1 PACKAGE 06/21/2020 20 37.8 mL in 1 POUCH; Type 0: Not a Combination Product 21 NDC:75109-503-21 25 in 1 PACKAGE 06/21/2020 21 94.5 mL in 1 POUCH; Type 0: Not a Combination Product 22 NDC:75109-503-22 50 in 1 PACKAGE 06/21/2020 22 189 mL in 1 POUCH; Type 0: Not a Combination Product 23 NDC:75109-503-23 70 in 1 CANISTER 06/21/2020 23 236.572 mL in 1 POUCH; Type 0: Not a Combination Product 24 NDC:75109-503-24 100 in 1 CANISTER 06/21/2020 24 186.2 mL in 1 POUCH; Type 0: Not a Combination Product 25 NDC:75109-503-25 800 in 1 PACKAGE 06/21/2020 25 1515.136 mL in 1 POUCH; Type 0: Not a Combination Product 26 NDC:75109-503-26 1200 in 1 PACKAGE 06/21/2020 26 2272.704 mL in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC monograph not final part333A 06/20/2020 Labeler - Kangna (Zhejiang) Medical Supplies Co., Ltd. (554530173) Establishment Name Address ID/FEI Business Operations Kangna (Zhejiang) Medical Supplies Co., Ltd. 554530173 manufacture(75109-503)