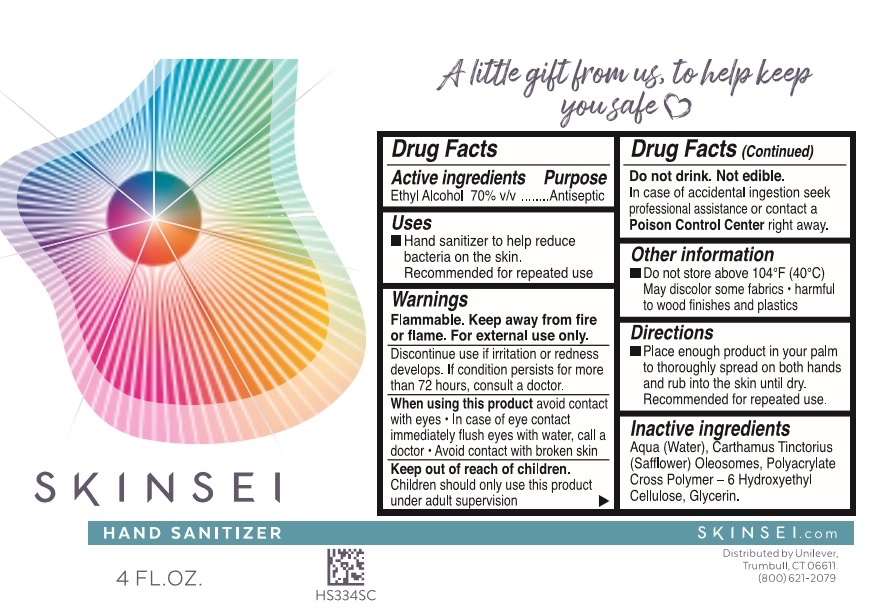
Label: SKINSEI- ethyl alcohol liquid liquid
- NDC Code(s): 64942-1740-1
- Packager: Conopco Inc. d/b/a/ Unilever
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated November 7, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SKINSEI HAND SANITIZER - Alcohol liquid
- Drug Facts
- Purpose
- Uses
-
Warnings
Flammable. Keep away from fire or flame
For external use only
Discontinue use if irritation or redness develops. If condition persists for more than 72 hours, consult a doctor.
When using this product avoid contact with eyes · In case of contact, flush eyes with water, call a doctor · Avoid contact with broken skinDo not drink. Not edible.
In case of accidental ingeston seek professional assistance or contact a Poison Contro Center right away. - KEEP OUT OF REACH OF CHILDREN
- Directions
- Other information
- Inactive ingredients
- QUESTIONS
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
SKINSEI
ethyl alcohol liquid liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:64942-1740 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength ALCOHOL (UNII: 3K9958V90M) (ALCOHOL - UNII:3K9958V90M) ALCOHOL 700 mg in 1 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) GLYCERIN (UNII: PDC6A3C0OX) CARTHAMUS TINCTORIUS SEED OLEOSOMES (UNII: 9S60Q72309) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:64942-1740-1 118 mL in 1 CONTAINER; Type 0: Not a Combination Product 06/01/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M016 06/01/2020 Labeler - Conopco Inc. d/b/a/ Unilever (001375088)