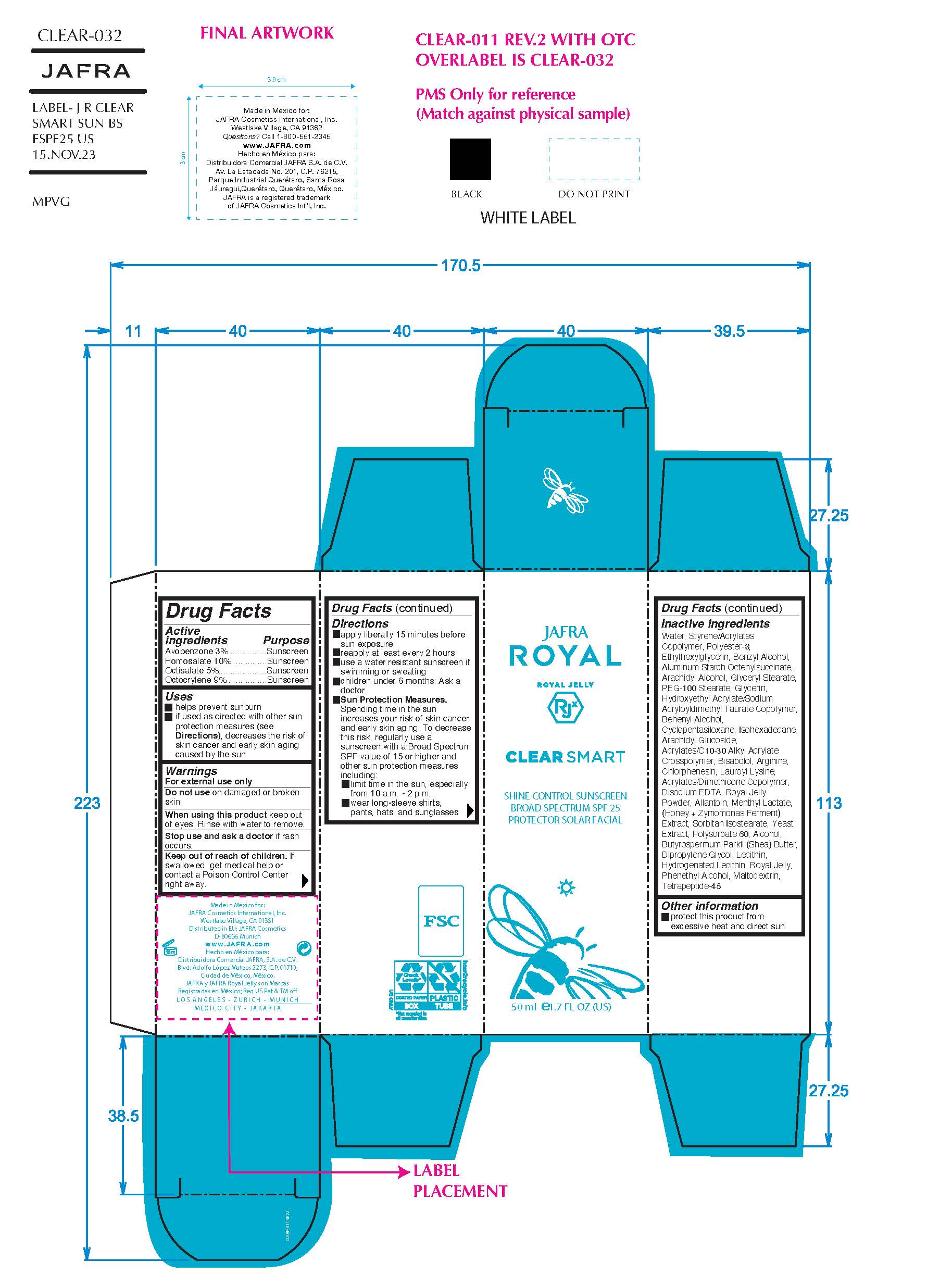
Label: CLEAR SMART SHINE CONTROL SUNSCREEN BROAD SPECTRUM SPF 25- avobenzone,homosalate,octisalate,octocrylene cream
- NDC Code(s): 68828-290-01, 68828-290-02
- Packager: Jafra Cosmetics International Inc
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated December 28, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- ACTIVE INGREDIENT
- Purpose
- Uses
- Warnings
- KEEP OUT OF REACH OF CHILDREN
-
Directions
- Apply liberally 15 minutes before sun exposure
- Use a water-resistant sunscreen if swimming or sweating
- Reapply at least every 2 hours.
- Children under 6 months of age: Ask a doctor
- Sun Protection Measures.Spending time in the sun increases your risk of skin cancer and early skin aging. To decrease this risk, regularly use a sunscreen with a Broad Spectrum SPF value of 15 or higher and other sun protection measures including:
• limit your time in the sun, especially from 10 a.m. – 2 p.m.
• wear long-sleeved shirts, pants, hats, and sunglasses
-
INACTIVE INGREDIENT
Inactive ingredients
Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Acrylates/Dimethicone Copolymer, Alcohol, Allantoin, Aluminum Starch Octenylsuccinate, Arachidyl Alcohol, Arachidyl Glucoside, Arginine, Behenyl Alcohol, Benzyl Alcohol, Bisabolol, Butyrospermum Parkii (Shea) Butter, Chlorphenesin, Cyclopentasiloxane, Dipropylene Glycol, Disodium EDTA, Ethylhexylglycerin, Glycerin, Glyceryl Stearate, Honey Extract, Hydrogenated Lecithin, Hydroxyethyl Acrylate/Sodium Acryloyldimethyl Taurate Copolymer, Isohexadecane, Lauroyl Lysine, Lecithin, Maltodextrin, Menthyl Lactate, PEG-100 Stearate, Phenethyl Alcohol, Polyester-8, Polysorbate 60, Royal Jelly, Royal Jelly Powder, Sorbitan Isostearate, Styrene/Acrylates Copolymer, Tetrapeptide-45, Water/Aqua, Yeast Extract, Zymomonas Ferment Extract
- Other information
- Product label
-
INGREDIENTS AND APPEARANCE
CLEAR SMART SHINE CONTROL SUNSCREEN BROAD SPECTRUM SPF 25
avobenzone,homosalate,octisalate,octocrylene creamProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:68828-290 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength AVOBENZONE (UNII: G63QQF2NOX) (AVOBENZONE - UNII:G63QQF2NOX) AVOBENZONE 3 g in 100 mL HOMOSALATE (UNII: V06SV4M95S) (HOMOSALATE - UNII:V06SV4M95S) HOMOSALATE 10 g in 100 mL OCTISALATE (UNII: 4X49Y0596W) (OCTISALATE - UNII:4X49Y0596W) OCTISALATE 5 g in 100 mL OCTOCRYLENE (UNII: 5A68WGF6WM) (OCTOCRYLENE - UNII:5A68WGF6WM) OCTOCRYLENE 9 g in 100 mL Inactive Ingredients Ingredient Name Strength MENTHYL LACTATE, (-)- (UNII: 2BF9E65L7I) ALLANTOIN (UNII: 344S277G0Z) ARGININE (UNII: 94ZLA3W45F) DOCOSANOL (UNII: 9G1OE216XY) CARBOMER COPOLYMER TYPE A (ALLYL PENTAERYTHRITOL CROSSLINKED) (UNII: 71DD5V995L) ETHYLHEXYLGLYCERIN (UNII: 147D247K3P) LEVOMENOL (UNII: 24WE03BX2T) WATER (UNII: 059QF0KO0R) LECITHIN, SOYBEAN (UNII: 1DI56QDM62) SHEA BUTTER (UNII: K49155WL9Y) GLYCERIN (UNII: PDC6A3C0OX) CYCLOMETHICONE 5 (UNII: 0THT5PCI0R) POLYESTER-8 (1400 MW, CYANODIPHENYLPROPENOYL CAPPED) (UNII: T9296U138P) ISOHEXADECANE (UNII: 918X1OUF1E) GLYCERYL MONOSTEARATE (UNII: 230OU9XXE4) POLYSORBATE 60 (UNII: CAL22UVI4M) BENZYL ALCOHOL (UNII: LKG8494WBH) EDETATE DISODIUM (UNII: 7FLD91C86K) PEG-100 STEARATE (UNII: YD01N1999R) HONEY (UNII: Y9H1V576FH) HYDROXYETHYL ACRYLATE/SODIUM ACRYLOYLDIMETHYL TAURATE COPOLYMER (100000 MPA.S AT 1.5%) (UNII: 86FQE96TZ4) DIPROPYLENE GLYCOL (UNII: E107L85C40) ROYAL JELLY (UNII: L497I37F0C) ALUMINUM STARCH OCTENYLSUCCINATE (UNII: I9PJ0O6294) ARACHIDYL ALCOHOL (UNII: 1QR1QRA9BU) ARACHIDYL GLUCOSIDE (UNII: 6JVW35JOOJ) HYDROGENATED SOYBEAN LECITHIN (UNII: H1109Z9J4N) MALTODEXTRIN (UNII: 7CVR7L4A2D) SORBITAN ISOSTEARATE (UNII: 01S2G2C1E4) ALCOHOL (UNII: 3K9958V90M) PHENYLETHYL ALCOHOL (UNII: ML9LGA7468) CHLORPHENESIN (UNII: I670DAL4SZ) LAUROYL LYSINE (UNII: 113171Q70B) STYRENE/ACRYLAMIDE COPOLYMER (500000 MW) (UNII: 5Z4DPO246A) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:68828-290-01 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 05/11/2020 09/15/2022 2 NDC:68828-290-02 50 mL in 1 TUBE; Type 0: Not a Combination Product 03/03/2022 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M020 05/11/2020 Labeler - Jafra Cosmetics International Inc (041676479) Establishment Name Address ID/FEI Business Operations Distribuidora Comercial Jafra, S.A. de C.V. 951612777 manufacture(68828-290)