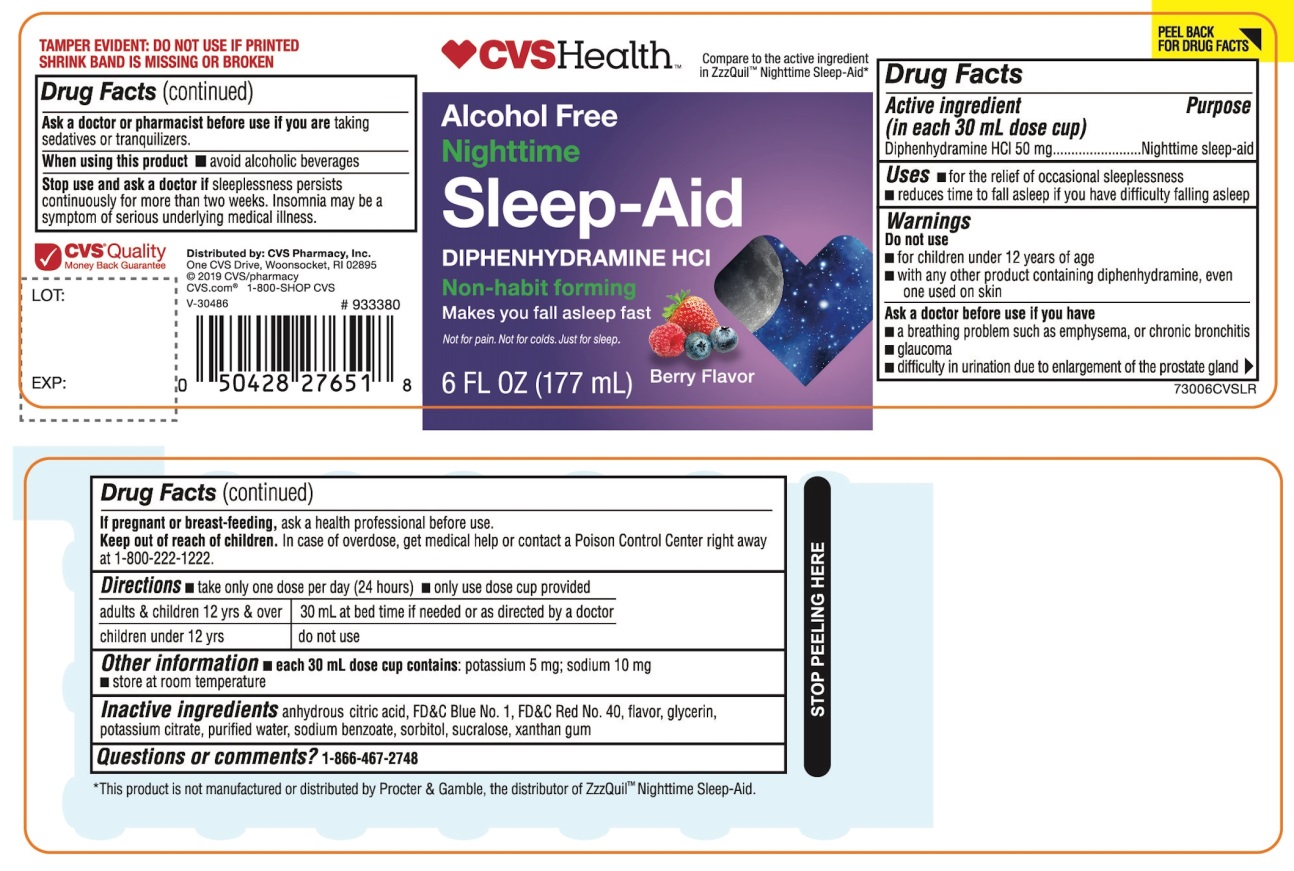
Label: CVS HEALTH NIGHTTIME SLEEP-AID- diphenhydramine hydrochloride liquid
- NDC Code(s): 59779-893-06
- Packager: CVS Pharmacy,Inc.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated August 21, 2024
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- SPL UNCLASSIFIED SECTION
- Active ingredient (in each 30 mL)
- Purpose
- Uses
-
Warnings
Do not use
- for children under 12 years of age
- with any other product containing diphenhydramine, even one used on skin
- with other drugs that cause drowsiness such as antihistamines and nighttime cold/flu products
Ask a doctor before use if you have
- a breathing problem such as asthma, emphysema, or chronic bronchitis
- glaucoma
- difficulty in urination due to enlargement of the prostate gland
- heart disease
Ask a doctor or pharmacist before use if you aretaking sedatives or tranquilizers or any other sleep- aid.
When using this product
- avoid alcoholic beverages and other drugs that cause drowsiness
- drowsiness will occur
- be careful when driving a motor vehicle or operating machinery
- Directions
- Other information
- Inactive ingredients
-
PRINCIPAL DISPLAY PANEL
CVS Health™
NDC 59779-893-06
Compare to the active ingredient in ZzzQuil® Nighttime Sleep-Aid*
Alcohol Free
NighttimeSleep-Aid
DIPHENHYDRAMINE HCl
Non-habit forming
- Not for treating cold or flu
Berry Flavor
Naturally and Artificially Flavored
6 FL OZ (177 mL)
FAILURE TO FOLLOW THESE WARNINGS COULD RESULT IN SERIOUS CONSEQUENCES.
TAMPER EVIDENT: DO NOT USE IF PRINTED SHRINK BAND IS MISSING OR BROKEN
*This product is not manufactured or distributed by Procter & Gamble, the distributor of ZzzQuil®
Distributed by: CVS Pharmacy, Inc.
One CVS Drive, Woonsocket, RI 02895
© 2019CVS/pharmacy
CVS.com ®1-800-SHOP CVS
V-30486
CVS® Quality
Money Back Guarantee
-
INGREDIENTS AND APPEARANCE
CVS HEALTH NIGHTTIME SLEEP-AID
diphenhydramine hydrochloride liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:59779-893 Route of Administration ORAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength DIPHENHYDRAMINE HYDROCHLORIDE (UNII: TC2D6JAD40) (DIPHENHYDRAMINE - UNII:8GTS82S83M) DIPHENHYDRAMINE HYDROCHLORIDE 50 mg in 30 mL Inactive Ingredients Ingredient Name Strength ANHYDROUS CITRIC ACID (UNII: XF417D3PSL) FD&C BLUE NO. 1 (UNII: H3R47K3TBD) FD&C RED NO. 40 (UNII: WZB9127XOA) GLYCERIN (UNII: PDC6A3C0OX) POTASSIUM CITRATE (UNII: EE90ONI6FF) WATER (UNII: 059QF0KO0R) SODIUM BENZOATE (UNII: OJ245FE5EU) SORBITOL (UNII: 506T60A25R) SUCRALOSE (UNII: 96K6UQ3ZD4) XANTHAN GUM (UNII: TTV12P4NEE) Product Characteristics Color purple Score Shape Size Flavor BERRY Imprint Code Contains Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:59779-893-06 177 mL in 1 BOTTLE, PLASTIC; Type 0: Not a Combination Product 09/22/2015 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M012 09/22/2015 Labeler - CVS Pharmacy,Inc. (062312574)