Label: WET WIPE- benzalkonium chloride swab
-
NDC Code(s):
76906-002-01,
76906-002-02,
76906-002-04,
76906-002-05, view more76906-002-06, 76906-002-07, 76906-002-08, 76906-002-09, 76906-002-10, 76906-002-11, 76906-002-12
- Packager: Yiwu Beitao Infant & Mother Supplies Co., Ltd.
- Category: HUMAN OTC DRUG LABEL
Drug Label Information
Updated July 15, 2020
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- Active Ingredient
- USE
- Warning
- Directions
- Inactive ingredients
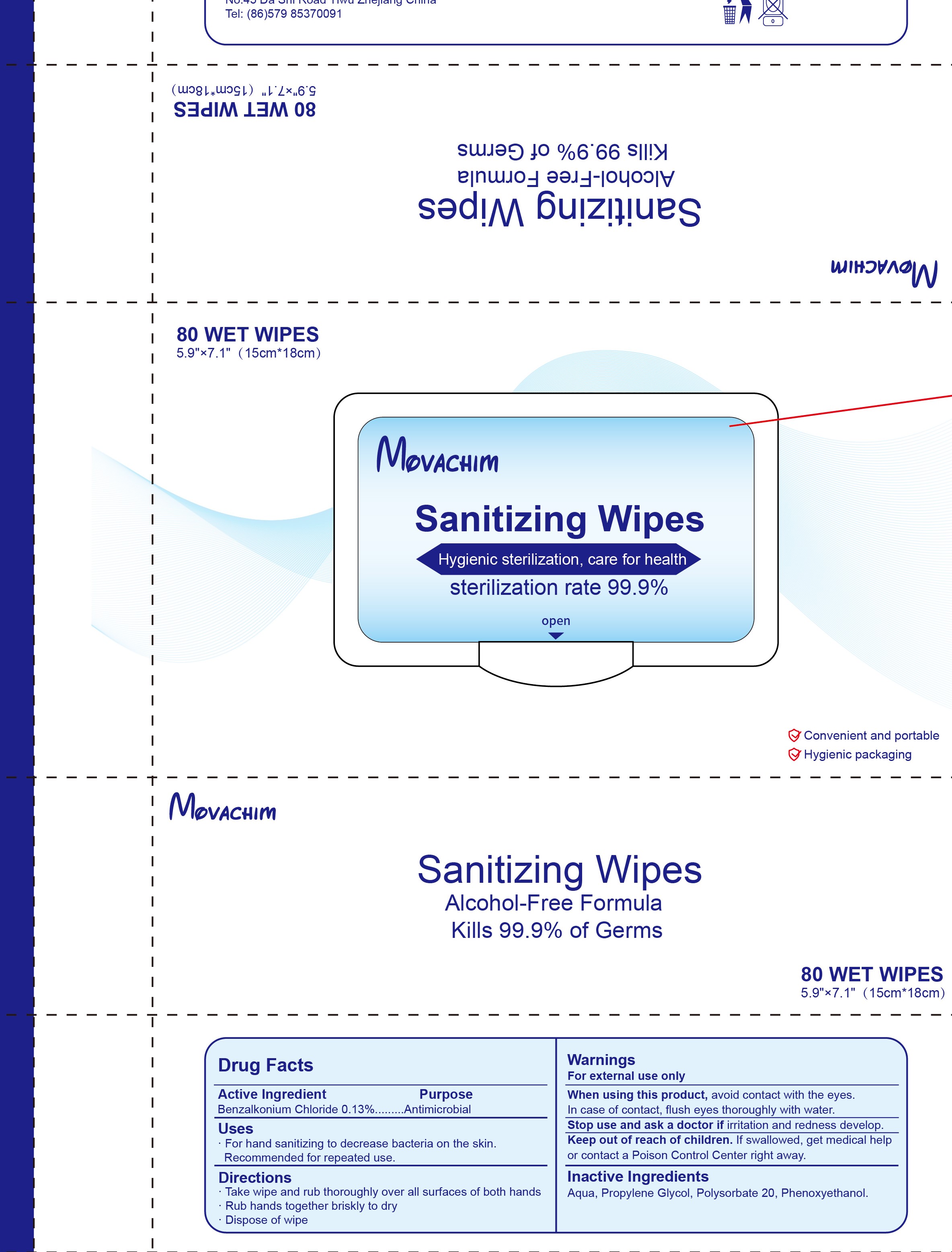
- PRINCIPAL DISPLAY PANEL
-
INGREDIENTS AND APPEARANCE
WET WIPE
benzalkonium chloride swabProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:76906-002 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength BENZALKONIUM CHLORIDE (UNII: F5UM2KM3W7) (BENZALKONIUM - UNII:7N6JUD5X6Y) BENZALKONIUM CHLORIDE 0.13 g in 100 g Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) POLYSORBATE 20 (UNII: 7T1F30V5YH) PROPYLENE GLYCOL (UNII: 6DC9Q167V3) PHENOXYETHANOL (UNII: HIE492ZZ3T) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:76906-002-07 100 in 1 BOX 03/21/2020 1 4.8 g in 1 POUCH; Type 0: Not a Combination Product 2 NDC:76906-002-08 135 in 1 PAIL 03/21/2020 2 2.07 g in 1 POUCH; Type 0: Not a Combination Product 3 NDC:76906-002-01 10 in 1 BAG 03/21/2020 3 5 g in 1 POUCH; Type 0: Not a Combination Product 4 NDC:76906-002-04 50 in 1 BAG 03/21/2020 4 6 g in 1 POUCH; Type 0: Not a Combination Product 5 NDC:76906-002-02 20 in 1 BAG 03/21/2020 5 6.5 g in 1 POUCH; Type 0: Not a Combination Product 6 NDC:76906-002-05 350 in 1 BAG 03/21/2020 6 5.8 g in 1 POUCH; Type 0: Not a Combination Product 7 NDC:76906-002-06 80 in 1 BAG 03/21/2020 7 5.25 g in 1 POUCH; Type 0: Not a Combination Product 8 NDC:76906-002-09 250 in 1 PAIL 03/21/2020 8 2.08 g in 1 POUCH; Type 0: Not a Combination Product 9 NDC:76906-002-10 60 in 1 PAIL 03/21/2020 9 5.83 g in 1 POUCH; Type 0: Not a Combination Product 10 NDC:76906-002-11 25 in 1 BAG 03/21/2020 10 8 g in 1 POUCH; Type 0: Not a Combination Product 11 NDC:76906-002-12 100 in 1 BAG 03/21/2020 11 4.8 g in 1 POUCH; Type 0: Not a Combination Product Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date OTC Monograph Drug M003 03/21/2020 Labeler - Yiwu Beitao Infant & Mother Supplies Co., Ltd. (412925352) Establishment Name Address ID/FEI Business Operations Yiwu Beitao Infant & Mother Supplies Co., Ltd. 412925352 manufacture(76906-002)