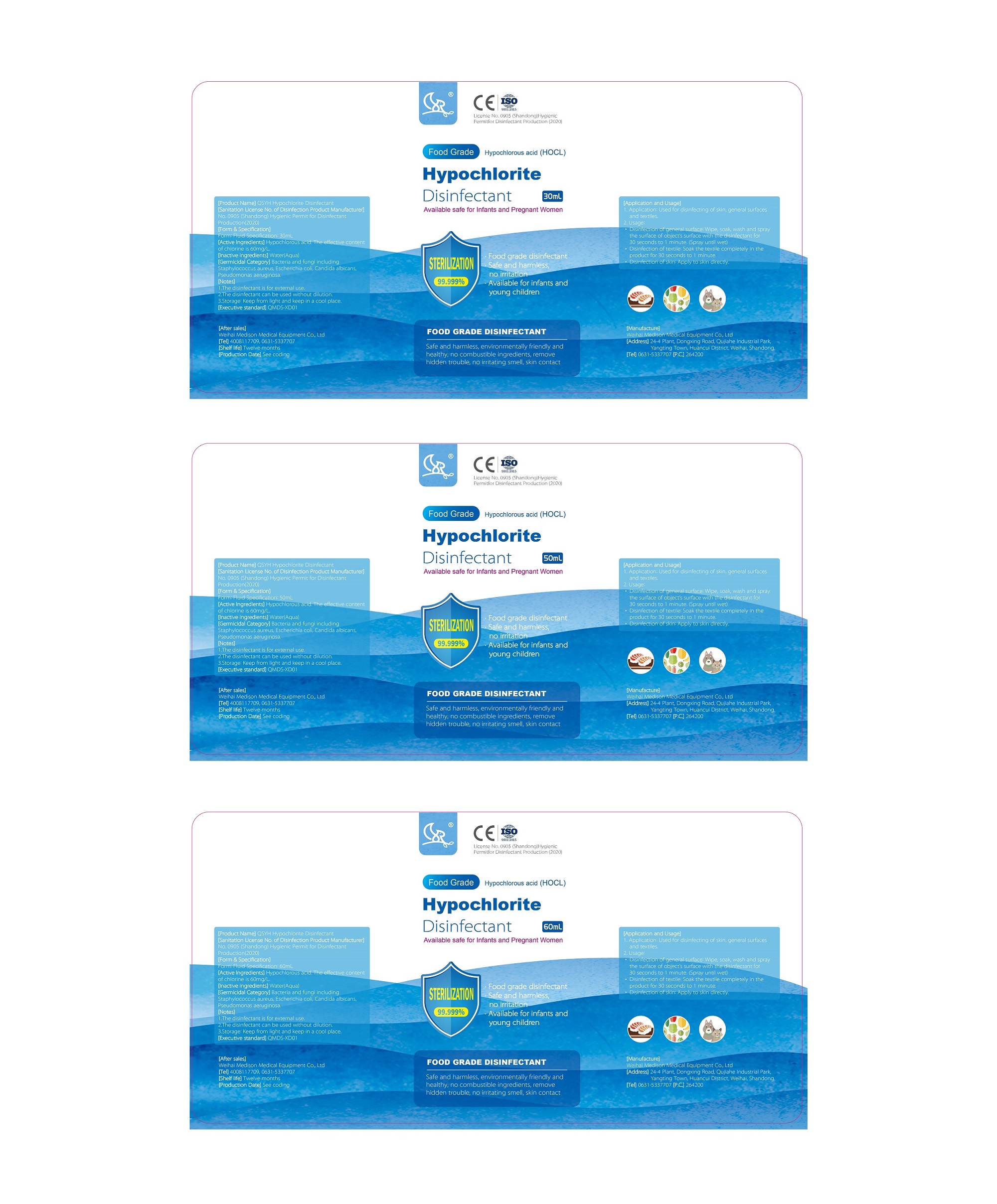
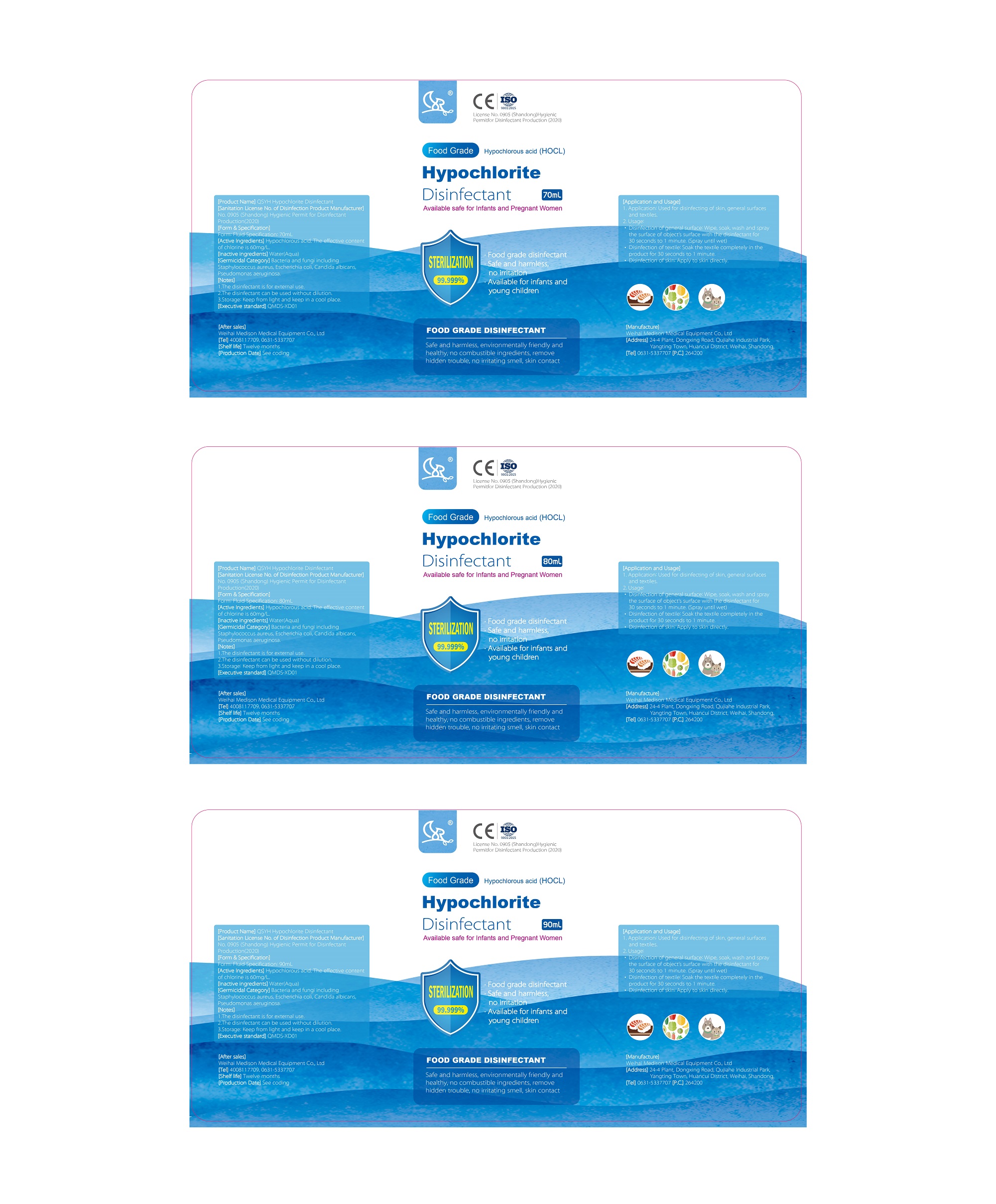
Label: QSYH HYPOCHLORITE DISINFECTANT liquid
-
NDC Code(s):
74744-005-01,
74744-005-02,
74744-005-03,
74744-005-04, view more74744-005-05, 74744-005-06, 74744-005-07, 74744-005-08, 74744-005-09, 74744-005-10, 74744-005-11, 74744-005-12, 74744-005-13, 74744-005-14, 74744-005-15, 74744-005-16, 74744-005-17, 74744-005-18, 74744-005-19, 74744-005-20, 74744-005-21, 74744-005-22, 74744-005-23, 74744-005-24, 74744-005-25, 74744-005-26, 74744-005-27, 74744-005-28
- Packager: Weihai Medison Medical Equipment Co., Ltd
- Category: HUMAN OTC DRUG LABEL
DISCLAIMER: This drug has not been found by FDA to be safe and effective, and this labeling has not been approved by FDA. For further information about unapproved drugs, click here.
Drug Label Information
Updated October 9, 2023
If you are a healthcare professional or from the pharmaceutical industry please visit this version.
- Download DRUG LABEL INFO: PDF XML
- Official Label (Printer Friendly)
- FOOD GRADE DISINFECTANT
- Active Ingredients
- Inactive ingredients
- Germicida Category
- Notes
- Application and Used
- Directions
- DO NOT USE
- WHEN USING
- STOP USE
- KEEP OUT OF REACH OF CHILDREN
- STORAGE AND HANDLING
- Package Label - Principal Display Panel
-
INGREDIENTS AND APPEARANCE
QSYH HYPOCHLORITE DISINFECTANT
qsyh hypochlorite disinfectant liquidProduct Information Product Type HUMAN OTC DRUG Item Code (Source) NDC:74744-005 Route of Administration TOPICAL Active Ingredient/Active Moiety Ingredient Name Basis of Strength Strength HYPOCHLOROUS ACID (UNII: 712K4CDC10) (HYPOCHLOROUS ACID - UNII:712K4CDC10) HYPOCHLOROUS ACID 0.006 g in 100 mL Inactive Ingredients Ingredient Name Strength WATER (UNII: 059QF0KO0R) Packaging # Item Code Package Description Marketing Start Date Marketing End Date 1 NDC:74744-005-01 30 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 2 NDC:74744-005-02 50 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 3 NDC:74744-005-03 60 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 4 NDC:74744-005-04 70 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 5 NDC:74744-005-05 80 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 6 NDC:74744-005-06 90 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 7 NDC:74744-005-07 100 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 8 NDC:74744-005-08 120 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 9 NDC:74744-005-09 150 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 10 NDC:74744-005-10 180 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 11 NDC:74744-005-11 200 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 12 NDC:74744-005-12 300 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 13 NDC:74744-005-13 500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 14 NDC:74744-005-14 1000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 15 NDC:74744-005-15 1500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 16 NDC:74744-005-16 2000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 17 NDC:74744-005-17 2500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 18 NDC:74744-005-18 3500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 19 NDC:74744-005-19 3000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 20 NDC:74744-005-20 3800 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 21 NDC:74744-005-21 4500 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 22 NDC:74744-005-22 5000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 23 NDC:74744-005-23 8000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 24 NDC:74744-005-24 10000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 25 NDC:74744-005-25 15000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 26 NDC:74744-005-26 20000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 27 NDC:74744-005-27 30000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 28 NDC:74744-005-28 50000 mL in 1 BOTTLE; Type 0: Not a Combination Product 04/28/2020 Marketing Information Marketing Category Application Number or Monograph Citation Marketing Start Date Marketing End Date unapproved drug other 04/26/2020 Labeler - Weihai Medison Medical Equipment Co., Ltd (529609518) Establishment Name Address ID/FEI Business Operations Weihai Medison Medical Equipment Co., Ltd 529609518 manufacture(74744-005)